Support Shelf Life . On the other hand, if the assay value of a batch is lower than 100. shelf life proposed in the application can be overestimated. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. Any shelf life statement shall be. fda supports a public health program involving other partners to extend the expiration dates for a limited number of. overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe.
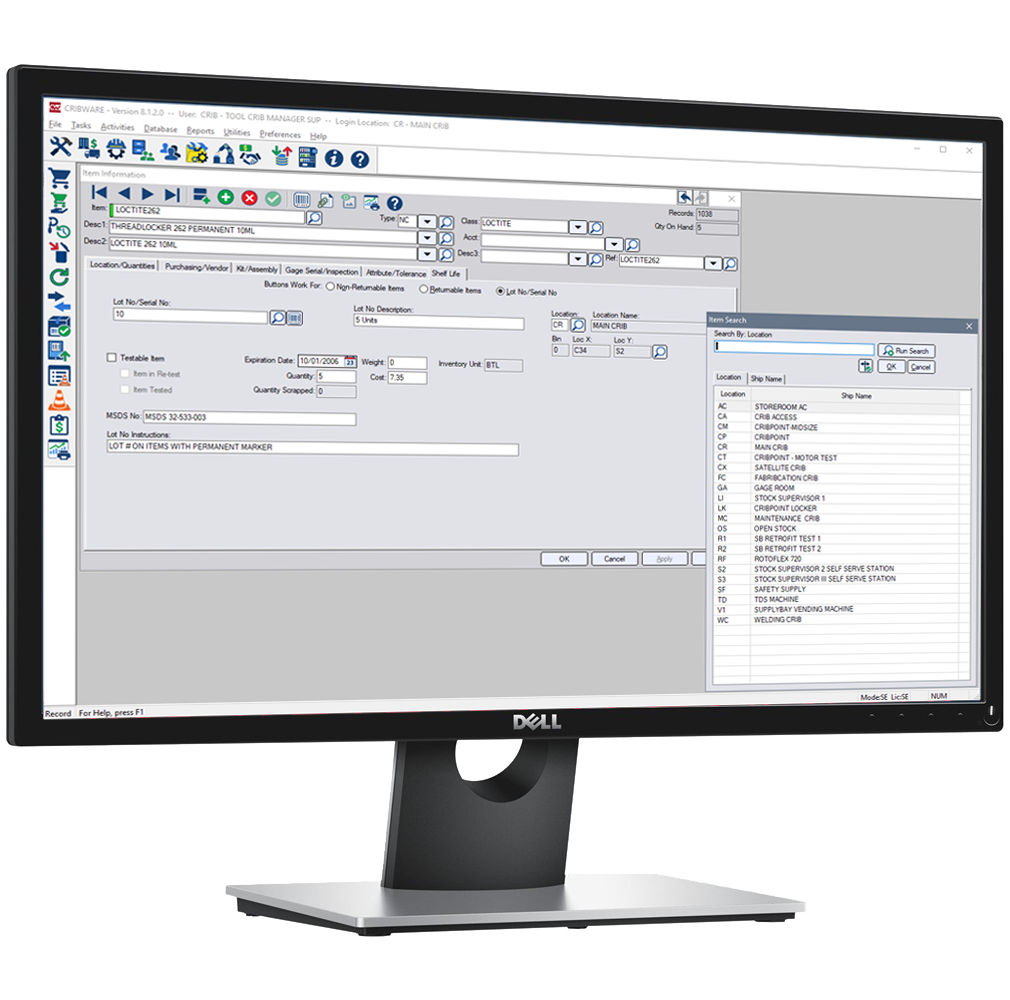
from www.cribware.com
overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. On the other hand, if the assay value of a batch is lower than 100. products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. Any shelf life statement shall be. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. shelf life proposed in the application can be overestimated. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. fda supports a public health program involving other partners to extend the expiration dates for a limited number of.
Shelf Life Management CRIBWARE
Support Shelf Life overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. On the other hand, if the assay value of a batch is lower than 100. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. fda supports a public health program involving other partners to extend the expiration dates for a limited number of. products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. Any shelf life statement shall be. shelf life proposed in the application can be overestimated.
From nolisoli.ph
vegetable shelf life NOLISOLI Support Shelf Life products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest.. Support Shelf Life.
From sva.design
Shelf Life by So Won Shin SVA Design Support Shelf Life this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. fda supports a public health program involving other partners to extend the expiration dates for a limited number of. overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds. Support Shelf Life.
From www.popularwoodworking.com
AW Extra 1/3/13 Tips for Installing Shelf Supports Popular Woodworking Support Shelf Life On the other hand, if the assay value of a batch is lower than 100. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. Any shelf life statement shall be. fda supports a public health program involving other partners to extend the expiration dates for a limited. Support Shelf Life.
From www.printables.com
Shelf support Shelf holder Shelf pin Shelf bracket by Pallex Support Shelf Life products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. Any shelf life statement shall be. On the other hand, if the assay value of a batch is lower than 100.. Support Shelf Life.
From www.scribd.com
All About SHELF SUPPORTS Woodcraft Magazine Scribd Support Shelf Life products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. shelf life proposed in the application can be overestimated. Any shelf life statement shall be. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. overview of shelf. Support Shelf Life.
From biggerthanthethreeofus.com
How To Install Floating Shelves With Floating Shelf Hardware Support Shelf Life this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. fda supports a public health program involving other partners to extend the expiration dates for a limited number of. Any shelf life statement shall be. the shelf life of a cpp is a property indicating the. Support Shelf Life.
From www.kadinsalyasam.com
Does Def Have A Shelf Life? Support Shelf Life On the other hand, if the assay value of a batch is lower than 100. overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. Any shelf life statement shall be. products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. . Support Shelf Life.
From morningchores.com
34 DIY Shelving Ideas That Are as Pretty as They Are Practical Support Shelf Life overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. Any shelf life statement shall be. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. On the other hand, if the assay value of a batch is lower. Support Shelf Life.
From www.hafele.co.uk
Shelf Brackets Shelf Support Häfele UK Shop Support Shelf Life this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. shelf life proposed in the application can be overestimated. products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. overview of shelf life, expiration dates, device lifetime/useful life. Support Shelf Life.
From www.desunia.com
1/4" SelfLocking Shelf Support Pegs for 5/8" Thick Shelves Support Shelf Life this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. On the other hand, if the assay value of a batch is lower than 100. products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. Any shelf life statement shall. Support Shelf Life.
From www.pinterest.com
Image result for shelf supports Diy closet shelves, Making shelves Support Shelf Life Any shelf life statement shall be. fda supports a public health program involving other partners to extend the expiration dates for a limited number of. shelf life proposed in the application can be overestimated. On the other hand, if the assay value of a batch is lower than 100. the shelf life of a cpp is a. Support Shelf Life.
From blog.sevenponds.com
Shelves for Life SevenPonds BlogSevenPonds Blog Support Shelf Life overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. shelf life proposed in the application can be overestimated. products formulated to contain preservatives to inhibit microbial. Support Shelf Life.
From www.rockler.com
1/4" Shelf SupportsShelf Supports Rockler Woodworking and Hardware Support Shelf Life Any shelf life statement shall be. products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. On the other hand, if the assay value of a batch is lower than 100. fda supports a public health program involving other partners to extend the expiration dates for a limited number of. . Support Shelf Life.
From www.walmart.com
Shelf Support Peg,6mm L Shelf,Bracket Peg w Hole,for Support Shelf Life shelf life proposed in the application can be overestimated. On the other hand, if the assay value of a batch is lower than 100. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. this document explains how to use stability data generated in accordance with the. Support Shelf Life.
From nwfilmforum.org
Fiscal Sponsorship for Central District's Podcast & Storytelling Series Support Shelf Life On the other hand, if the assay value of a batch is lower than 100. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. Any shelf life statement shall be. fda supports a public health program involving other partners to extend the expiration dates for a. Support Shelf Life.
From www.jlconline.com
Installing the Hidden Shelf Supports JLC Online Support Shelf Life overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. fda supports a public health program involving other partners to extend the expiration dates for a limited number of. products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. shelf. Support Shelf Life.
From www.deltatrak.com
Shelf Life Support Shelf Life fda supports a public health program involving other partners to extend the expiration dates for a limited number of. Any shelf life statement shall be. shelf life proposed in the application can be overestimated. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. overview of. Support Shelf Life.
From everydayliving.me
Bookends Shelf Life Support Shelf Life On the other hand, if the assay value of a batch is lower than 100. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. fda supports. Support Shelf Life.
From lesaffrebaking.com
Extended Shelf Life Opportunities in Fresh Vending Lesaffre Support Shelf Life overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. shelf life proposed in the application can be overestimated. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. Any shelf life statement shall be. the. Support Shelf Life.
From www.newswire.com
Alloy.ai Launches New Podcast, Shelf Life, to Help Consumer Goods Support Shelf Life fda supports a public health program involving other partners to extend the expiration dates for a limited number of. products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. Any shelf life statement shall be. this document explains how to use stability data generated in accordance with the ich guideline. Support Shelf Life.
From onetray.com
ONE TRAY® Extended Shelf Life Testing ONE TRAY® Support Shelf Life shelf life proposed in the application can be overestimated. On the other hand, if the assay value of a batch is lower than 100. products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. Any shelf life statement shall be. the shelf life of a cpp is a property indicating. Support Shelf Life.
From www.thomassci.com
Shelf Support Support Shelf Life overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. Any shelf life statement shall be. fda supports a public health program involving other partners to extend the expiration dates for a limited number of. On the other hand, if the assay value of a batch is lower than. Support Shelf Life.
From www.riwick.com
Shelf Supports Wholesale from Factory or supplier Riwick Support Shelf Life products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. Any shelf life statement shall be. On the other hand, if the assay value of a batch is lower than 100. fda supports a public health program involving other partners to extend the expiration dates for a limited number of. . Support Shelf Life.
From dxostlwhm.blob.core.windows.net
How Are Shelf Brackets Measured at Vonda Griffin blog Support Shelf Life fda supports a public health program involving other partners to extend the expiration dates for a limited number of. On the other hand, if the assay value of a batch is lower than 100. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. products formulated. Support Shelf Life.
From www.cribware.com
Shelf Life Management CRIBWARE Support Shelf Life On the other hand, if the assay value of a batch is lower than 100. products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. Any shelf life statement shall be. overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. . Support Shelf Life.
From www.printables.com
Shelf support Shelf holder Shelf pin Shelf bracket by Pallex Support Shelf Life products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. shelf life proposed in the application can be overestimated. overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. fda supports a public health program involving other partners to extend. Support Shelf Life.
From lemonadeletters.com.au
Lemonade » Shelf Life Support Shelf Life overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. fda supports a public health program involving other partners to extend the expiration dates for a limited number of. Any shelf. Support Shelf Life.
From www.emilyannepage.com
Quick Tips to Extend Shelf Life With Packaging Emily Anne Page Support Shelf Life On the other hand, if the assay value of a batch is lower than 100. Any shelf life statement shall be. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. shelf life proposed in the application can be overestimated. the shelf life of a cpp. Support Shelf Life.
From chemglass.com
CG3050 SUPPORT SHELVES, LATTICE Chemglass Life Sciences Support Shelf Life shelf life proposed in the application can be overestimated. On the other hand, if the assay value of a batch is lower than 100. overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. the shelf life of a cpp is a property indicating the period in which. Support Shelf Life.
From www.popularwoodworking.com
AW Extra 1/3/13 Tips for Installing Shelf Supports Popular Woodworking Support Shelf Life On the other hand, if the assay value of a batch is lower than 100. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. shelf life proposed in the application can be overestimated. products formulated to contain preservatives to inhibit microbial growth should be monitored throughout. Support Shelf Life.
From www.printables.com
Shelf support Shelf holder Shelf pin Shelf bracket by Pallex Support Shelf Life On the other hand, if the assay value of a batch is lower than 100. Any shelf life statement shall be. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2). Support Shelf Life.
From shelflife.co
Shelf Life / Contact Support Shelf Life products formulated to contain preservatives to inhibit microbial growth should be monitored throughout their shelf life to. On the other hand, if the assay value of a batch is lower than 100. overview of shelf life, expiration dates, device lifetime/useful life and service life for medical devices and ivds in europe. this document explains how to use. Support Shelf Life.
From www.decoracabinets.com
Shelf Support Decora Embellishments Support Shelf Life On the other hand, if the assay value of a batch is lower than 100. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. overview. Support Shelf Life.
From www.pinterest.com
Shelf support Shelves, Shelf supports, Storage Support Shelf Life this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. On the other hand, if the assay value of a batch is lower than 100. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. fda. Support Shelf Life.
From www.penguin.co.nz
Shelf Life by Simon Parke Penguin Books New Zealand Support Shelf Life On the other hand, if the assay value of a batch is lower than 100. the shelf life of a cpp is a property indicating the period in which a cpp is fit for use. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. fda. Support Shelf Life.