What Substances Are The Most Soluble In Water . We’re going to go over what solubility is, how it works, and the complete list of solubility rules to help you determine the solubility of substances. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. It is a polar compound capable of forming hydrogen bonds and dissolving several. substances that dissolve in water to yield ions are called electrolytes. These rules are general and qualitative in nature. a table for the solubility of salts in water. Nonelectrolytes are substances that do not produce ions. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. The determining factor for the result is the. when a substance is mixed with a solvent, there are several possible results. water is the most widely used solvent due to its excellent properties. The salts of alkali metals are soluble.
from studylib.net
substances that dissolve in water to yield ions are called electrolytes. It is a polar compound capable of forming hydrogen bonds and dissolving several. We’re going to go over what solubility is, how it works, and the complete list of solubility rules to help you determine the solubility of substances. substances that dissolve in water to yield ions are called electrolytes. The salts of alkali metals are soluble. These rules are general and qualitative in nature. a table for the solubility of salts in water. Nonelectrolytes are substances that do not produce ions. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. Nonelectrolytes are substances that do not produce ions.
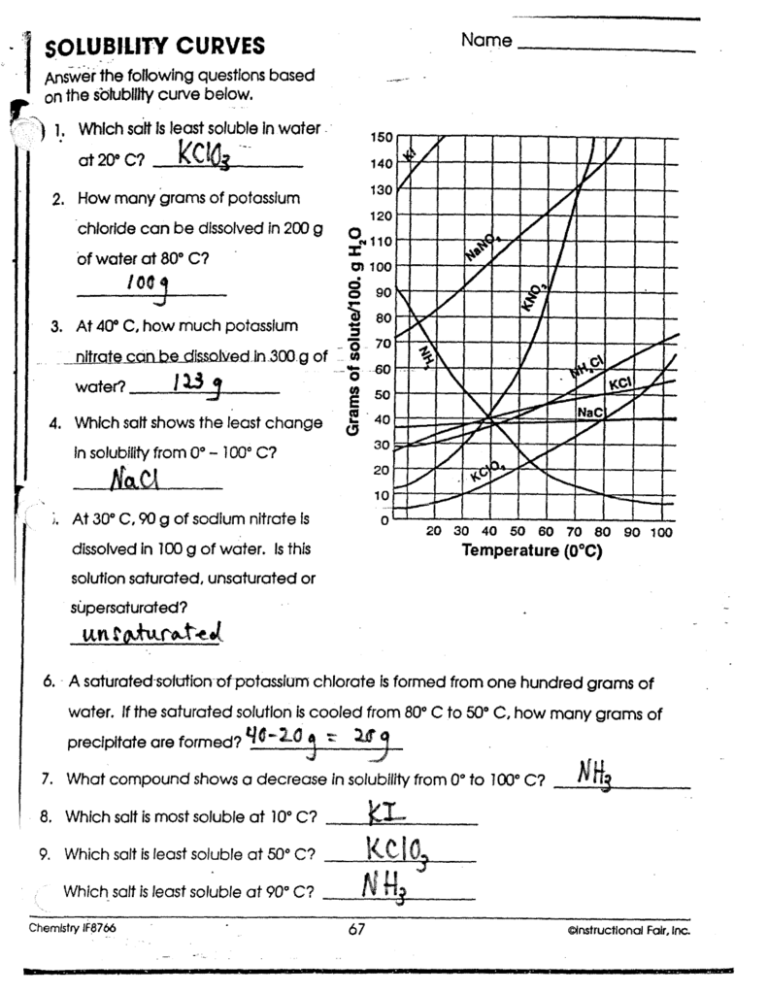
solubility curves
What Substances Are The Most Soluble In Water Nonelectrolytes are substances that do not produce ions. The determining factor for the result is the. Nonelectrolytes are substances that do not produce ions. These rules are general and qualitative in nature. We’re going to go over what solubility is, how it works, and the complete list of solubility rules to help you determine the solubility of substances. substances that dissolve in water to yield ions are called electrolytes. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. The salts of alkali metals are soluble. substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions. It is a polar compound capable of forming hydrogen bonds and dissolving several. when a substance is mixed with a solvent, there are several possible results. water is the most widely used solvent due to its excellent properties. a table for the solubility of salts in water.
From www.alamy.com
Solution science experiment. Solubility of salt and sand in water What Substances Are The Most Soluble In Water These rules are general and qualitative in nature. substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions. The determining factor for the result is the. when a substance is mixed with a solvent, there are several possible results. Nonelectrolytes are substances that do not produce ions. water. What Substances Are The Most Soluble In Water.
From socratic.org
Why are metallic compounds insoluble in water? Socratic What Substances Are The Most Soluble In Water substances that dissolve in water to yield ions are called electrolytes. when a substance is mixed with a solvent, there are several possible results. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. The determining factor for the result is the. These rules are general and qualitative in nature. water is the most widely used. What Substances Are The Most Soluble In Water.
From www.coursehero.com
[Solved] how to determine most soluble in water? Course Hero What Substances Are The Most Soluble In Water The salts of alkali metals are soluble. The determining factor for the result is the. substances that dissolve in water to yield ions are called electrolytes. We’re going to go over what solubility is, how it works, and the complete list of solubility rules to help you determine the solubility of substances. substances that dissolve in water to. What Substances Are The Most Soluble In Water.
From oneclass.com
OneClass Rank the following substances in order from most soluble in What Substances Are The Most Soluble In Water The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. We’re going to go over what solubility is, how it works, and the complete list of solubility rules to help you determine the solubility of substances. substances that dissolve in water to yield ions are called electrolytes. water is the most widely used solvent due to its. What Substances Are The Most Soluble In Water.
From www.crestolympiads.com
Solute, Solvent, and Solution Notes Science Olympiad Class 4 What Substances Are The Most Soluble In Water The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. These rules are general and qualitative in nature. a table for the solubility of salts in water. substances that dissolve in water to yield ions are called electrolytes. when a substance is mixed with a solvent, there are several possible results. The determining factor for the. What Substances Are The Most Soluble In Water.
From rayb78.github.io
Solubility In Water Chart What Substances Are The Most Soluble In Water Nonelectrolytes are substances that do not produce ions. We’re going to go over what solubility is, how it works, and the complete list of solubility rules to help you determine the solubility of substances. substances that dissolve in water to yield ions are called electrolytes. substances that dissolve in water to yield ions are called electrolytes. The salts. What Substances Are The Most Soluble In Water.
From www.youtube.com
which molecule is most soluble in water? YouTube What Substances Are The Most Soluble In Water a table for the solubility of salts in water. when a substance is mixed with a solvent, there are several possible results. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. These rules are general and qualitative in nature. It is a polar compound capable of forming hydrogen bonds and dissolving several. Nonelectrolytes are substances that. What Substances Are The Most Soluble In Water.
From www.sliderbase.com
Solutions and solubility Presentation Chemistry What Substances Are The Most Soluble In Water The salts of alkali metals are soluble. when a substance is mixed with a solvent, there are several possible results. These rules are general and qualitative in nature. Nonelectrolytes are substances that do not produce ions. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. Nonelectrolytes are substances that do not produce ions. a table for. What Substances Are The Most Soluble In Water.
From alfonsoewtfry.blogspot.com
Is Salt Soluble in Water AlfonsoewtFry What Substances Are The Most Soluble In Water We’re going to go over what solubility is, how it works, and the complete list of solubility rules to help you determine the solubility of substances. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. The salts of alkali metals are soluble. a table for the solubility of. What Substances Are The Most Soluble In Water.
From ar.inspiredpencil.com
Solubility In Water What Substances Are The Most Soluble In Water water is the most widely used solvent due to its excellent properties. The determining factor for the result is the. It is a polar compound capable of forming hydrogen bonds and dissolving several. a table for the solubility of salts in water. These rules are general and qualitative in nature. Nonelectrolytes are substances that do not produce ions.. What Substances Are The Most Soluble In Water.
From www.numerade.com
SOLVED Which compound is the MOST soluble in water? OH "OH What Substances Are The Most Soluble In Water Nonelectrolytes are substances that do not produce ions. a table for the solubility of salts in water. water is the most widely used solvent due to its excellent properties. when a substance is mixed with a solvent, there are several possible results. The salts of alkali metals are soluble. substances that dissolve in water to yield. What Substances Are The Most Soluble In Water.
From www.expii.com
Soluble vs. Insoluble — Comparison & Examples Expii What Substances Are The Most Soluble In Water water is the most widely used solvent due to its excellent properties. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. substances that dissolve in water to yield ions are called electrolytes. These rules are general and qualitative in nature. The determining factor for the result is the. We’re going to go over what solubility is,. What Substances Are The Most Soluble In Water.
From socratic.org
Substance A will not dissolve in water. What can be said about What Substances Are The Most Soluble In Water The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. The salts of alkali metals are soluble. Nonelectrolytes are substances that do not produce ions. Nonelectrolytes are substances that do not produce ions. a table for the solubility of salts in water. substances that dissolve in water to yield ions are called electrolytes. The determining factor for. What Substances Are The Most Soluble In Water.
From www.chegg.com
Solved Which compound will be the most soluble in water? What Substances Are The Most Soluble In Water We’re going to go over what solubility is, how it works, and the complete list of solubility rules to help you determine the solubility of substances. Nonelectrolytes are substances that do not produce ions. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. substances that dissolve in water to yield ions are called electrolytes. These rules are. What Substances Are The Most Soluble In Water.
From dxonxarcb.blob.core.windows.net
What Type Of Substances Will Dissolve In Water To Form Aqueous What Substances Are The Most Soluble In Water Nonelectrolytes are substances that do not produce ions. It is a polar compound capable of forming hydrogen bonds and dissolving several. These rules are general and qualitative in nature. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. a table for the solubility of salts in water. Nonelectrolytes are substances that do not produce ions. substances. What Substances Are The Most Soluble In Water.
From www.pinterest.com.mx
Solubility Rules Chart for Chemistry Classroom Teaching chemistry What Substances Are The Most Soluble In Water substances that dissolve in water to yield ions are called electrolytes. water is the most widely used solvent due to its excellent properties. These rules are general and qualitative in nature. Nonelectrolytes are substances that do not produce ions. It is a polar compound capable of forming hydrogen bonds and dissolving several. The determining factor for the result. What Substances Are The Most Soluble In Water.
From www.chegg.com
Solved Rank The Following Substances In Order From Most S... What Substances Are The Most Soluble In Water when a substance is mixed with a solvent, there are several possible results. It is a polar compound capable of forming hydrogen bonds and dissolving several. substances that dissolve in water to yield ions are called electrolytes. a table for the solubility of salts in water. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),.. What Substances Are The Most Soluble In Water.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube What Substances Are The Most Soluble In Water Nonelectrolytes are substances that do not produce ions. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. The determining factor for the result is the. a table for the solubility of salts in water. These rules are general and qualitative in nature. substances that dissolve in water. What Substances Are The Most Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID2493612 What Substances Are The Most Soluble In Water The salts of alkali metals are soluble. substances that dissolve in water to yield ions are called electrolytes. a table for the solubility of salts in water. substances that dissolve in water to yield ions are called electrolytes. We’re going to go over what solubility is, how it works, and the complete list of solubility rules to. What Substances Are The Most Soluble In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry What Substances Are The Most Soluble In Water The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. substances that dissolve in water to yield ions are called electrolytes. The salts of alkali metals are soluble. water is the most widely used solvent due to its excellent properties. These rules are general and qualitative in nature. We’re going to go over what solubility is, how. What Substances Are The Most Soluble In Water.
From www.youtube.com
L8 Common soluble and insoluble substances, objects that float or sink What Substances Are The Most Soluble In Water The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. It is a polar compound capable of forming hydrogen bonds and dissolving several. The determining factor for the result is the. a table for the solubility of salts in water. We’re going to go over what solubility is, how it works, and the complete list of solubility rules. What Substances Are The Most Soluble In Water.
From www.polyemat.com
What You Need To Know About WaterSoluble Material Polyemat What Substances Are The Most Soluble In Water a table for the solubility of salts in water. These rules are general and qualitative in nature. It is a polar compound capable of forming hydrogen bonds and dissolving several. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. Nonelectrolytes are substances that do not produce ions. We’re going to go over what solubility is, how it. What Substances Are The Most Soluble In Water.
From study.com
Compound Solubility in Water Overview & Examples Lesson What Substances Are The Most Soluble In Water The salts of alkali metals are soluble. when a substance is mixed with a solvent, there are several possible results. substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. It is a polar compound capable of. What Substances Are The Most Soluble In Water.
From gracelimlf.blogspot.com
LIM LAI FONG (D20102043844) Science Year 3 What Substances Are The Most Soluble In Water These rules are general and qualitative in nature. substances that dissolve in water to yield ions are called electrolytes. when a substance is mixed with a solvent, there are several possible results. The determining factor for the result is the. Nonelectrolytes are substances that do not produce ions. water is the most widely used solvent due to. What Substances Are The Most Soluble In Water.
From www.dreamstime.com
Solubility Vector Illustration. Labeled Solute, Solvent and Solution What Substances Are The Most Soluble In Water Nonelectrolytes are substances that do not produce ions. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. when a substance is mixed with a solvent, there are several possible results. It is a polar compound capable of forming hydrogen bonds and dissolving several. These rules are general and. What Substances Are The Most Soluble In Water.
From www.thoughtco.com
Solubility Definition (Chemistry) What Substances Are The Most Soluble In Water The determining factor for the result is the. These rules are general and qualitative in nature. We’re going to go over what solubility is, how it works, and the complete list of solubility rules to help you determine the solubility of substances. It is a polar compound capable of forming hydrogen bonds and dissolving several. Nonelectrolytes are substances that do. What Substances Are The Most Soluble In Water.
From dxogtzdjb.blob.core.windows.net
Copper Hydroxide Is Soluble In Water at Linwood blog What Substances Are The Most Soluble In Water when a substance is mixed with a solvent, there are several possible results. Nonelectrolytes are substances that do not produce ions. Nonelectrolytes are substances that do not produce ions. It is a polar compound capable of forming hydrogen bonds and dissolving several. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. These rules are general and qualitative. What Substances Are The Most Soluble In Water.
From rayb78.github.io
Solubility In Water Chart What Substances Are The Most Soluble In Water substances that dissolve in water to yield ions are called electrolytes. substances that dissolve in water to yield ions are called electrolytes. water is the most widely used solvent due to its excellent properties. The determining factor for the result is the. It is a polar compound capable of forming hydrogen bonds and dissolving several. These rules. What Substances Are The Most Soluble In Water.
From solutionpharmacy.in
Factors Influencing Solubility Solution Parmacy What Substances Are The Most Soluble In Water These rules are general and qualitative in nature. Nonelectrolytes are substances that do not produce ions. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. substances that dissolve in water to yield ions are called electrolytes. The salts of alkali metals are soluble. water is the most widely used solvent due to its excellent properties. . What Substances Are The Most Soluble In Water.
From studylib.net
solubility curves What Substances Are The Most Soluble In Water Nonelectrolytes are substances that do not produce ions. The determining factor for the result is the. Nonelectrolytes are substances that do not produce ions. We’re going to go over what solubility is, how it works, and the complete list of solubility rules to help you determine the solubility of substances. These rules are general and qualitative in nature. substances. What Substances Are The Most Soluble In Water.
From www.chegg.com
Solved Rank the following substances in order from most What Substances Are The Most Soluble In Water Nonelectrolytes are substances that do not produce ions. We’re going to go over what solubility is, how it works, and the complete list of solubility rules to help you determine the solubility of substances. It is a polar compound capable of forming hydrogen bonds and dissolving several. water is the most widely used solvent due to its excellent properties.. What Substances Are The Most Soluble In Water.
From www.chegg.com
Solved Part A Rank the following substances in order from What Substances Are The Most Soluble In Water when a substance is mixed with a solvent, there are several possible results. The salts of alkali metals are soluble. substances that dissolve in water to yield ions are called electrolytes. We’re going to go over what solubility is, how it works, and the complete list of solubility rules to help you determine the solubility of substances. . What Substances Are The Most Soluble In Water.
From www.youtube.com
Solubility in Water Chapter4 Sorting Materials into groups Class What Substances Are The Most Soluble In Water a table for the solubility of salts in water. when a substance is mixed with a solvent, there are several possible results. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. substances that dissolve in water to yield ions are called electrolytes. The salts of alkali metals are soluble. The determining factor for the result. What Substances Are The Most Soluble In Water.
From www.youtube.com
water soluble substances and water insoluble substances YouTube What Substances Are The Most Soluble In Water when a substance is mixed with a solvent, there are several possible results. water is the most widely used solvent due to its excellent properties. The determining factor for the result is the. Nonelectrolytes are substances that do not produce ions. We’re going to go over what solubility is, how it works, and the complete list of solubility. What Substances Are The Most Soluble In Water.
From brainly.com
Which of the following substances are likely to be soluble in water? A What Substances Are The Most Soluble In Water substances that dissolve in water to yield ions are called electrolytes. water is the most widely used solvent due to its excellent properties. The hydroxides of alkaline earth metals, like magnesium (mg 2+ ),. It is a polar compound capable of forming hydrogen bonds and dissolving several. The salts of alkali metals are soluble. We’re going to go. What Substances Are The Most Soluble In Water.