Tryptophan Fluorescence . the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. learn how to use tryptophan fluorescence to study protein structure and function without modification. in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. these studies provide a framework for deriving detailed structural and dynamical information from. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal.
from www3.nd.edu
in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. learn how to use tryptophan fluorescence to study protein structure and function without modification. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal. the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. these studies provide a framework for deriving detailed structural and dynamical information from.
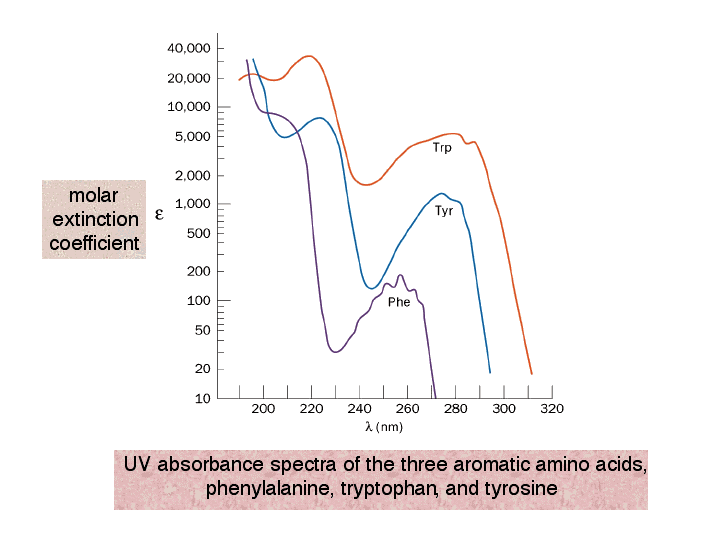
UV absorbance spectra of the three aromatic amino acids, phenylalanine, tryptophan, and tyrosine
Tryptophan Fluorescence this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. learn how to use tryptophan fluorescence to study protein structure and function without modification. the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. these studies provide a framework for deriving detailed structural and dynamical information from. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the.
From www.researchgate.net
Tryptophan fluorescence binding assay shows the binding strength of the... Download Scientific Tryptophan Fluorescence we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal. the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. these studies provide a framework for deriving detailed structural and dynamical information from. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs. Tryptophan Fluorescence.
From www.researchgate.net
Intrinsic tryptophan fluorescence spectra of D1 PSI at pH 4, 5, 6, and... Download Scientific Tryptophan Fluorescence the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. these studies provide a framework for deriving detailed structural and dynamical information from. in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. we have predicted the fluorescence wavelengths of 19 tryptophans in 16. Tryptophan Fluorescence.
From www3.nd.edu
UV absorbance spectra of the three aromatic amino acids, phenylalanine, tryptophan, and tyrosine Tryptophan Fluorescence learn how to use tryptophan fluorescence to study protein structure and function without modification. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. these studies provide a framework for deriving detailed structural and dynamical information from. the ubiquitous presence of tryptophan residues in the crds of all members of human. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence assay showed rapid assembly. Assembly was... Download Scientific Diagram Tryptophan Fluorescence learn how to use tryptophan fluorescence to study protein structure and function without modification. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. this review describes the application of intrinsic protein fluorescence, predominantly. Tryptophan Fluorescence.
From www.researchgate.net
Changes in tryptophan fluorescence allow monitoring of FfhFtsY complex... Download Scientific Tryptophan Fluorescence we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal. in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. this review describes the application of intrinsic protein fluorescence, predominantly derived. Tryptophan Fluorescence.
From www.researchgate.net
Intrinsic tryptophan fluorescence emission spectra (uncorrected) of... Download Scientific Diagram Tryptophan Fluorescence the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. these studies provide a framework for deriving detailed structural and dynamical information from. learn how to use tryptophan fluorescence to study protein structure and function without modification. in this article, we systematically describe the most common applications in fluorescence. Tryptophan Fluorescence.
From www.pnas.org
Sitedirected tryptophan fluorescence reveals two essential conformational changes in the Na+/H+ Tryptophan Fluorescence these studies provide a framework for deriving detailed structural and dynamical information from. learn how to use tryptophan fluorescence to study protein structure and function without modification. the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins,. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence images at a depth of 0, 5, 15, 30, 30, and 70... Download Scientific Tryptophan Fluorescence this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. these studies provide a framework for deriving detailed structural and. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence spectra of temporin L in an aqueous buffer ()... Download Scientific Tryptophan Fluorescence learn how to use tryptophan fluorescence to study protein structure and function without modification. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting. Tryptophan Fluorescence.
From www.researchgate.net
Protein interaction with ligands monitored by the Tryptophan... Download Scientific Diagram Tryptophan Fluorescence the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal. these studies provide a framework for deriving detailed structural. Tryptophan Fluorescence.
From dxozosgyd.blob.core.windows.net
Tryptophan Fluorescence Quenching at Charles Larsen blog Tryptophan Fluorescence this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. learn how to use tryptophan fluorescence to study protein structure and function without modification. these studies provide a framework for deriving detailed structural and dynamical. Tryptophan Fluorescence.
From www.researchgate.net
Quenching of the intrinsic tryptophan fluorescence of SoPIP2;1 at 335... Download Scientific Tryptophan Fluorescence in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. these studies provide a framework for deriving detailed structural and dynamical information. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence intensity measurement of HγDcrys samples (0.1... Download Scientific Tryptophan Fluorescence the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. these studies provide a framework for deriving detailed structural and dynamical information from. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins,. Tryptophan Fluorescence.
From www.researchgate.net
Intrinsic tryptophan fluorescence spectra at various pressures and... Download Scientific Diagram Tryptophan Fluorescence the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. learn how to use tryptophan fluorescence to study protein structure and function without modification. in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. these studies provide a framework for deriving detailed structural and. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence lifetime distributions of wildtype yPGK with... Download Scientific Tryptophan Fluorescence tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. learn how to use tryptophan fluorescence to study protein structure and function without modification. the ubiquitous presence of tryptophan residues in the crds of all members of. Tryptophan Fluorescence.
From dxohkuzts.blob.core.windows.net
Fluorescence Detection Tryptophan at Irwin Follis blog Tryptophan Fluorescence these studies provide a framework for deriving detailed structural and dynamical information from. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with. Tryptophan Fluorescence.
From www.researchgate.net
Representative fluorescent emission spectra of tryptophan. The... Download Scientific Diagram Tryptophan Fluorescence learn how to use tryptophan fluorescence to study protein structure and function without modification. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting. Tryptophan Fluorescence.
From www.nature.com
Functional dynamics of a single tryptophan residue in a BLUF protein revealed by fluorescence Tryptophan Fluorescence the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. this review describes the application of intrinsic protein fluorescence,. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence emission spectra of antithrombin in the absence... Download Scientific Tryptophan Fluorescence this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. learn how to use tryptophan fluorescence to study protein structure and function without modification. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits. Tryptophan Fluorescence.
From mediomics.com
BridgeIt® LTryptophan Fluorescence Assay Kit, 384well format Mediomics Tryptophan Fluorescence the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. these studies provide a framework for deriving detailed structural and dynamical information from. we have predicted the fluorescence wavelengths of 19 tryptophans in 16. Tryptophan Fluorescence.
From www.researchgate.net
Intrinsic tryptophan fluorescence study of AbnU contributed by W114... Download Scientific Diagram Tryptophan Fluorescence this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. these studies provide a framework for deriving detailed structural and dynamical information from. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with. Tryptophan Fluorescence.
From www.researchgate.net
Figure S1 Normalized Intrinsic tryptophan fluorescence emission... Download Scientific Diagram Tryptophan Fluorescence learn how to use tryptophan fluorescence to study protein structure and function without modification. these studies provide a framework for deriving detailed structural and dynamical information from. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ.. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence quenching of TbGMPR W115R by purine... Download Scientific Diagram Tryptophan Fluorescence the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. in this article, we systematically describe the most common. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence analysis of peptides. Tryptophan fluorescence... Download Scientific Tryptophan Fluorescence we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal. the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. learn how to use tryptophan fluorescence to study protein. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence measurement for the determination of metal ion... Download Scientific Tryptophan Fluorescence this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. these studies provide a framework for deriving detailed structural and. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence data for the MIT domain. A Spectra in 50 mM... Download Scientific Tryptophan Fluorescence in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. learn how to use tryptophan fluorescence to study protein. Tryptophan Fluorescence.
From pubs.acs.org
Insights into the Mechanism of Tryptophan Fluorescence Quenching due to Synthetic Crowding Tryptophan Fluorescence learn how to use tryptophan fluorescence to study protein structure and function without modification. these studies provide a framework for deriving detailed structural and dynamical information from. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal.. Tryptophan Fluorescence.
From www.edinst.com
Tryptophan Fluorescence as a Research and Diagnostics Tool Tryptophan Fluorescence we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal. the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. learn how to use tryptophan fluorescence to study protein structure and function without modification. this review describes the application of intrinsic protein fluorescence, predominantly. Tryptophan Fluorescence.
From www.researchgate.net
Fluorescence emission spectra of enzymes having a single tryptophan... Download Scientific Diagram Tryptophan Fluorescence these studies provide a framework for deriving detailed structural and dynamical information from. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. the ubiquitous presence of tryptophan residues in the crds of all members of human. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence intensity at the tryptophan emission maximum... Download Scientific Tryptophan Fluorescence this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. learn how to use tryptophan fluorescence to study protein structure and function without modification. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal. the ubiquitous presence of tryptophan residues in the crds of all members of. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence spectra of WT FXN (1.4 μM) and SOD2 (1.9 μM).... Download Scientific Tryptophan Fluorescence in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. learn how to use tryptophan fluorescence to study protein structure and function without modification. these studies provide a framework for deriving detailed structural and dynamical information from. we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence spectra of UVB irradiated purified Download Scientific Tryptophan Fluorescence tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. learn how to use tryptophan fluorescence to study protein structure and function without modification. the ubiquitous presence of tryptophan residues in the crds of all members of. Tryptophan Fluorescence.
From www.cell.com
Mechanisms of Tryptophan Fluorescence Shifts in Proteins Biophysical Journal Tryptophan Fluorescence these studies provide a framework for deriving detailed structural and dynamical information from. in this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins,. learn how to use tryptophan fluorescence to study protein structure and function without modification. tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in. Tryptophan Fluorescence.
From www.researchgate.net
Tryptophan fluorescence spectra of high salt treated purified... Download Scientific Diagram Tryptophan Fluorescence tryptophan, the most commonly used fluorophore among natural amino acids, absorbs and emits in the. the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. this review describes the application of intrinsic protein fluorescence, predominantly derived from tryptophan (λ. we have predicted the fluorescence wavelengths of 19 tryptophans in. Tryptophan Fluorescence.
From www.mdpi.com
IJMS Free FullText Intrinsic Tryptophan Fluorescence in the Detection and Analysis of Tryptophan Fluorescence we have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal. the ubiquitous presence of tryptophan residues in the crds of all members of human galectins and the. these studies provide a framework for deriving detailed structural and dynamical information from. learn how to use tryptophan fluorescence to study protein structure and. Tryptophan Fluorescence.