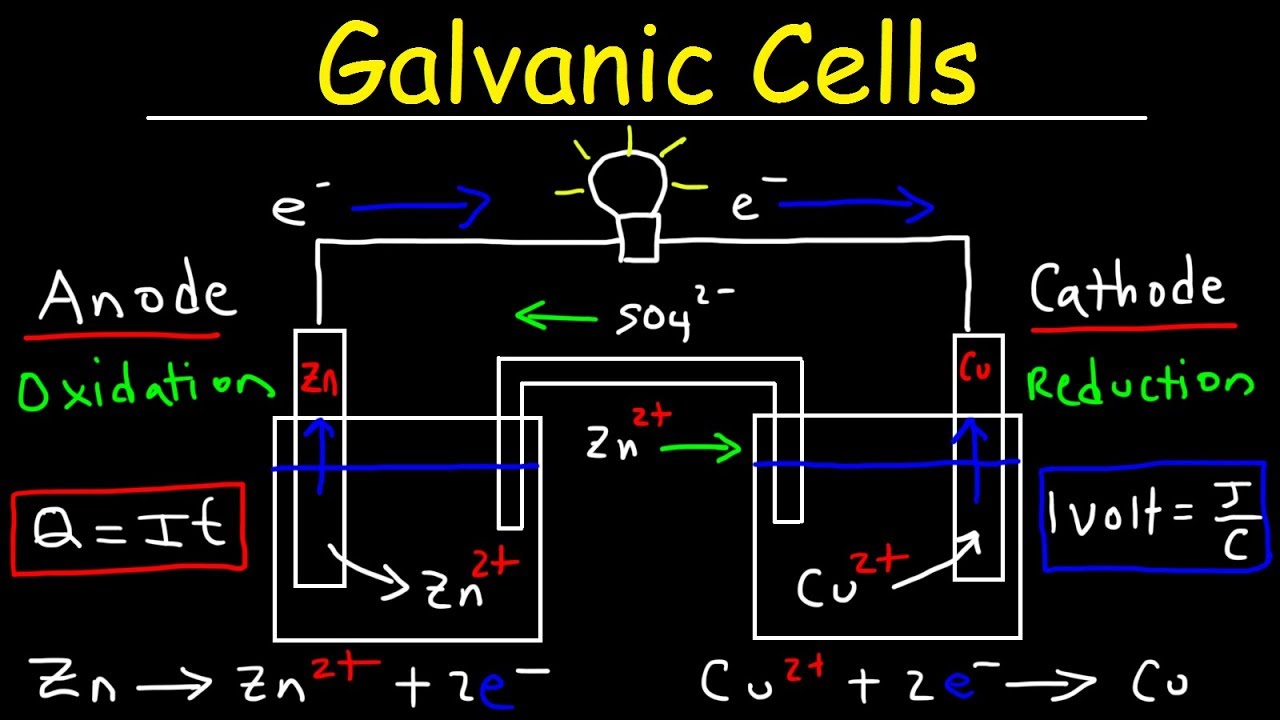
Examples Of Galvanic Cell And Electrolytic Cell . In a galvanic cell, spontaneous chemical. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. There are two types of electrochemical cells: In writing the equations, it is often. An electrolytic cell hosts a nonspontaneous reaction which requires. Galvanic and electrolytic cells are two types of electrochemical cells. Electrochemical cells are devices that convert chemical energy into electrical energy through. A galvanic cell hosts a spontaneous reaction which produces chemical energy. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. Two common types of electrochemical cells are electrolytic cells and galvanic cells. Galvanic cells and electrolytic cells.
from mungfali.com
A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. There are two types of electrochemical cells: Two common types of electrochemical cells are electrolytic cells and galvanic cells. A galvanic cell hosts a spontaneous reaction which produces chemical energy. In writing the equations, it is often. An electrolytic cell hosts a nonspontaneous reaction which requires. Galvanic cells and electrolytic cells. Galvanic and electrolytic cells are two types of electrochemical cells. Electrochemical cells are devices that convert chemical energy into electrical energy through. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart.
Galvanic And Electrolytic Cells
Examples Of Galvanic Cell And Electrolytic Cell An electrolytic cell hosts a nonspontaneous reaction which requires. A galvanic cell hosts a spontaneous reaction which produces chemical energy. In a galvanic cell, spontaneous chemical. An electrolytic cell hosts a nonspontaneous reaction which requires. Galvanic and electrolytic cells are two types of electrochemical cells. There are two types of electrochemical cells: Electrochemical cells are devices that convert chemical energy into electrical energy through. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. Galvanic cells and electrolytic cells. In writing the equations, it is often. Two common types of electrochemical cells are electrolytic cells and galvanic cells.
From pediaa.com
Difference Between Galvanic and Electrolytic Cell Definition, How Examples Of Galvanic Cell And Electrolytic Cell A galvanic cell hosts a spontaneous reaction which produces chemical energy. Two common types of electrochemical cells are electrolytic cells and galvanic cells. An electrolytic cell hosts a nonspontaneous reaction which requires. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. A galvanic cell is an electrochemical cell in which. Examples Of Galvanic Cell And Electrolytic Cell.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Examples Of Galvanic Cell And Electrolytic Cell A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. A galvanic cell hosts a spontaneous reaction which produces chemical energy. An electrolytic cell hosts a nonspontaneous reaction which requires. Electrochemical cells are devices that convert chemical energy into electrical energy through. Galvanic cells and electrolytic cells. Two common types. Examples Of Galvanic Cell And Electrolytic Cell.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902 Examples Of Galvanic Cell And Electrolytic Cell An electrolytic cell hosts a nonspontaneous reaction which requires. Two common types of electrochemical cells are electrolytic cells and galvanic cells. Galvanic cells and electrolytic cells. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. In writing the equations, it is often. A galvanic cell is an electrochemical cell in. Examples Of Galvanic Cell And Electrolytic Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Examples Of Galvanic Cell And Electrolytic Cell In a galvanic cell, spontaneous chemical. An electrolytic cell hosts a nonspontaneous reaction which requires. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. There are two types of electrochemical cells: Two common types of electrochemical cells are electrolytic cells and galvanic cells. Galvanic and electrolytic cells are two. Examples Of Galvanic Cell And Electrolytic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Examples Of Galvanic Cell And Electrolytic Cell There are two types of electrochemical cells: A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. A galvanic cell hosts a spontaneous reaction which produces chemical energy. In writing the equations, it is often. Two common types of electrochemical cells are electrolytic cells and galvanic cells. Electrochemical cells are. Examples Of Galvanic Cell And Electrolytic Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5404890 Examples Of Galvanic Cell And Electrolytic Cell Electrochemical cells are devices that convert chemical energy into electrical energy through. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. Galvanic cells and electrolytic cells. A galvanic cell hosts a spontaneous reaction which produces chemical energy. Two common types of electrochemical cells are electrolytic cells and galvanic cells. An. Examples Of Galvanic Cell And Electrolytic Cell.
From www.goconqr.com
Electrochemistry Mind Map Examples Of Galvanic Cell And Electrolytic Cell Galvanic and electrolytic cells are two types of electrochemical cells. Galvanic cells and electrolytic cells. An electrolytic cell hosts a nonspontaneous reaction which requires. Two common types of electrochemical cells are electrolytic cells and galvanic cells. A galvanic cell hosts a spontaneous reaction which produces chemical energy. In writing the equations, it is often. Electrochemical cells are devices that convert. Examples Of Galvanic Cell And Electrolytic Cell.
From www.pinterest.pt
Galvanic Cell and Electrolytic Cell Electrochemical Cells Teaching Examples Of Galvanic Cell And Electrolytic Cell Galvanic and electrolytic cells are two types of electrochemical cells. In a galvanic cell, spontaneous chemical. Galvanic cells and electrolytic cells. A galvanic cell hosts a spontaneous reaction which produces chemical energy. An electrolytic cell hosts a nonspontaneous reaction which requires. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart.. Examples Of Galvanic Cell And Electrolytic Cell.
From courses.lumenlearning.com
Galvanic Cells Chemistry Examples Of Galvanic Cell And Electrolytic Cell Galvanic cells and electrolytic cells. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. In a galvanic cell, spontaneous chemical. Two common types of electrochemical cells are electrolytic cells and galvanic cells. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set. Examples Of Galvanic Cell And Electrolytic Cell.
From psiberg.com
Galvanic vs. Electrolytic Cell The Two Types of Electrochemical Cells Examples Of Galvanic Cell And Electrolytic Cell In writing the equations, it is often. An electrolytic cell hosts a nonspontaneous reaction which requires. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. Galvanic cells and electrolytic cells.. Examples Of Galvanic Cell And Electrolytic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Examples Of Galvanic Cell And Electrolytic Cell Electrochemical cells are devices that convert chemical energy into electrical energy through. An electrolytic cell hosts a nonspontaneous reaction which requires. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. In writing the equations, it is often. In a galvanic cell, spontaneous chemical. There are two types of electrochemical cells:. Examples Of Galvanic Cell And Electrolytic Cell.
From www.youtube.com
Galvanic Cell Definition, Construction, Working, Example, Diagram Examples Of Galvanic Cell And Electrolytic Cell Galvanic cells and electrolytic cells. In a galvanic cell, spontaneous chemical. An electrolytic cell hosts a nonspontaneous reaction which requires. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. Galvanic and electrolytic cells are two types of electrochemical cells. There are two types of electrochemical cells: A galvanic cell hosts. Examples Of Galvanic Cell And Electrolytic Cell.
From employees.csbsju.edu
CHEM 123 GENERAL CHEMISTRY 1 Examples Of Galvanic Cell And Electrolytic Cell Electrochemical cells are devices that convert chemical energy into electrical energy through. An electrolytic cell hosts a nonspontaneous reaction which requires. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. Galvanic and electrolytic cells are two types of electrochemical cells. In a galvanic cell, spontaneous chemical. A galvanic cell hosts. Examples Of Galvanic Cell And Electrolytic Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Examples Of Galvanic Cell And Electrolytic Cell An electrolytic cell hosts a nonspontaneous reaction which requires. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. Two common types of electrochemical cells are electrolytic cells and galvanic cells. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart.. Examples Of Galvanic Cell And Electrolytic Cell.
From www.youtube.com
Galvanic versus Electrolytic Cells YouTube Examples Of Galvanic Cell And Electrolytic Cell Galvanic cells and electrolytic cells. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. An electrolytic cell hosts a nonspontaneous reaction which requires. In a galvanic cell, spontaneous chemical. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. There. Examples Of Galvanic Cell And Electrolytic Cell.
From dxoufitvg.blob.core.windows.net
What's The Difference Between Galvanic And Electrochemical Cells at Examples Of Galvanic Cell And Electrolytic Cell A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. There are two types of electrochemical cells: While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. Electrochemical cells are devices that convert chemical energy into electrical energy through. An electrolytic. Examples Of Galvanic Cell And Electrolytic Cell.
From www.pinterest.com
Galvanic Cell or Voltaic cell Construction and working Examples Of Galvanic Cell And Electrolytic Cell Two common types of electrochemical cells are electrolytic cells and galvanic cells. A galvanic cell hosts a spontaneous reaction which produces chemical energy. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. Galvanic cells and electrolytic cells. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur. Examples Of Galvanic Cell And Electrolytic Cell.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell Examples Of Galvanic Cell And Electrolytic Cell While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. There are two types of electrochemical cells: An electrolytic cell hosts a nonspontaneous reaction which requires. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. Electrochemical cells are devices that. Examples Of Galvanic Cell And Electrolytic Cell.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter Examples Of Galvanic Cell And Electrolytic Cell An electrolytic cell hosts a nonspontaneous reaction which requires. Electrochemical cells are devices that convert chemical energy into electrical energy through. There are two types of electrochemical cells: Two common types of electrochemical cells are electrolytic cells and galvanic cells. A galvanic cell hosts a spontaneous reaction which produces chemical energy. In writing the equations, it is often. Galvanic and. Examples Of Galvanic Cell And Electrolytic Cell.
From chem.libretexts.org
Electrolytic Cells Chemistry LibreTexts Examples Of Galvanic Cell And Electrolytic Cell An electrolytic cell hosts a nonspontaneous reaction which requires. Galvanic and electrolytic cells are two types of electrochemical cells. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. There are two types of electrochemical cells: In a galvanic cell, spontaneous chemical. While both cells involve redox reactions and the. Examples Of Galvanic Cell And Electrolytic Cell.
From www.alamy.com
Electrolysis process. galvanic cell element. Experiment with Examples Of Galvanic Cell And Electrolytic Cell Galvanic and electrolytic cells are two types of electrochemical cells. Two common types of electrochemical cells are electrolytic cells and galvanic cells. There are two types of electrochemical cells: Galvanic cells and electrolytic cells. In writing the equations, it is often. In a galvanic cell, spontaneous chemical. While both cells involve redox reactions and the flow of electrons, they have. Examples Of Galvanic Cell And Electrolytic Cell.
From www.javatpoint.com
Difference Between Galvanic Cells and Electrolytic Cells javatpoint Examples Of Galvanic Cell And Electrolytic Cell A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. Galvanic and electrolytic cells are two types of electrochemical cells. An electrolytic cell hosts a nonspontaneous reaction which requires. In writing the equations, it is often. Electrochemical cells are devices that convert chemical energy into electrical energy through. In a. Examples Of Galvanic Cell And Electrolytic Cell.
From electrochemistryh5t34.blogspot.com
We Love Electrochemistry Galvanic Cell Examples Of Galvanic Cell And Electrolytic Cell An electrolytic cell hosts a nonspontaneous reaction which requires. There are two types of electrochemical cells: Electrochemical cells are devices that convert chemical energy into electrical energy through. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. A galvanic cell hosts a spontaneous reaction which produces chemical energy. While. Examples Of Galvanic Cell And Electrolytic Cell.
From www.studypool.com
SOLUTION Electrochemical cells galvanic and electrolytic cell Studypool Examples Of Galvanic Cell And Electrolytic Cell Galvanic and electrolytic cells are two types of electrochemical cells. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. There are two types of electrochemical cells: In writing the equations,. Examples Of Galvanic Cell And Electrolytic Cell.
From in.pinterest.com
three different types of electronic devices are shown in this diagram Examples Of Galvanic Cell And Electrolytic Cell In writing the equations, it is often. There are two types of electrochemical cells: Galvanic and electrolytic cells are two types of electrochemical cells. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. Two common types of electrochemical cells are electrolytic cells and galvanic cells. In a galvanic cell,. Examples Of Galvanic Cell And Electrolytic Cell.
From www.alamy.com
Simple electrochemical or galvanic cell. The Daniell cell Stock Photo Examples Of Galvanic Cell And Electrolytic Cell While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. Two common types of electrochemical cells are electrolytic cells and galvanic cells. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. Electrochemical cells are devices that convert chemical energy into. Examples Of Galvanic Cell And Electrolytic Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Examples Of Galvanic Cell And Electrolytic Cell There are two types of electrochemical cells: In writing the equations, it is often. In a galvanic cell, spontaneous chemical. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through. Electrochemical. Examples Of Galvanic Cell And Electrolytic Cell.
From www.youtube.com
Galvanic Cells and Electrolytic Cells AP Chem YouTube Examples Of Galvanic Cell And Electrolytic Cell A galvanic cell hosts a spontaneous reaction which produces chemical energy. An electrolytic cell hosts a nonspontaneous reaction which requires. Galvanic cells and electrolytic cells. Electrochemical cells are devices that convert chemical energy into electrical energy through. In a galvanic cell, spontaneous chemical. Galvanic and electrolytic cells are two types of electrochemical cells. A galvanic cell is an electrochemical cell. Examples Of Galvanic Cell And Electrolytic Cell.
From www.youtube.com
GALVANIC CELL V/S COMMON ELECTROLYTIC CELL YouTube Examples Of Galvanic Cell And Electrolytic Cell While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. Galvanic cells and electrolytic cells. In a galvanic cell, spontaneous chemical. A galvanic cell hosts a spontaneous reaction which produces chemical energy. Two common types of electrochemical cells are electrolytic cells and galvanic cells. There are two types of electrochemical cells:. Examples Of Galvanic Cell And Electrolytic Cell.
From www.geeksforgeeks.org
Electrochemical Cell Definition, Types, Working Principle & Uses Examples Of Galvanic Cell And Electrolytic Cell Electrochemical cells are devices that convert chemical energy into electrical energy through. In a galvanic cell, spontaneous chemical. In writing the equations, it is often. A galvanic cell hosts a spontaneous reaction which produces chemical energy. Two common types of electrochemical cells are electrolytic cells and galvanic cells. While both cells involve redox reactions and the flow of electrons, they. Examples Of Galvanic Cell And Electrolytic Cell.
From mungfali.com
Galvanic And Electrolytic Cells Examples Of Galvanic Cell And Electrolytic Cell An electrolytic cell hosts a nonspontaneous reaction which requires. In a galvanic cell, spontaneous chemical. Galvanic and electrolytic cells are two types of electrochemical cells. Galvanic cells and electrolytic cells. There are two types of electrochemical cells: In writing the equations, it is often. Electrochemical cells are devices that convert chemical energy into electrical energy through. While both cells involve. Examples Of Galvanic Cell And Electrolytic Cell.
From www.vecteezy.com
Electrochemical cell or Galvanic cell, The Daniell cell 18989226 Vector Examples Of Galvanic Cell And Electrolytic Cell Galvanic cells and electrolytic cells. An electrolytic cell hosts a nonspontaneous reaction which requires. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. In writing the equations, it is often. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through.. Examples Of Galvanic Cell And Electrolytic Cell.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Examples Of Galvanic Cell And Electrolytic Cell In a galvanic cell, spontaneous chemical. An electrolytic cell hosts a nonspontaneous reaction which requires. In writing the equations, it is often. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. Two common types of electrochemical cells are electrolytic cells and galvanic cells. Galvanic cells and electrolytic cells. A galvanic. Examples Of Galvanic Cell And Electrolytic Cell.
From www.slideserve.com
PPT Galvanic and Electrolytic Cells PowerPoint Presentation, free Examples Of Galvanic Cell And Electrolytic Cell Galvanic and electrolytic cells are two types of electrochemical cells. An electrolytic cell hosts a nonspontaneous reaction which requires. A galvanic cell hosts a spontaneous reaction which produces chemical energy. In a galvanic cell, spontaneous chemical. Two common types of electrochemical cells are electrolytic cells and galvanic cells. While both cells involve redox reactions and the flow of electrons, they. Examples Of Galvanic Cell And Electrolytic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Examples Of Galvanic Cell And Electrolytic Cell In a galvanic cell, spontaneous chemical. Two common types of electrochemical cells are electrolytic cells and galvanic cells. Galvanic cells and electrolytic cells. An electrolytic cell hosts a nonspontaneous reaction which requires. In writing the equations, it is often. While both cells involve redox reactions and the flow of electrons, they have distinct attributes that set them apart. There are. Examples Of Galvanic Cell And Electrolytic Cell.