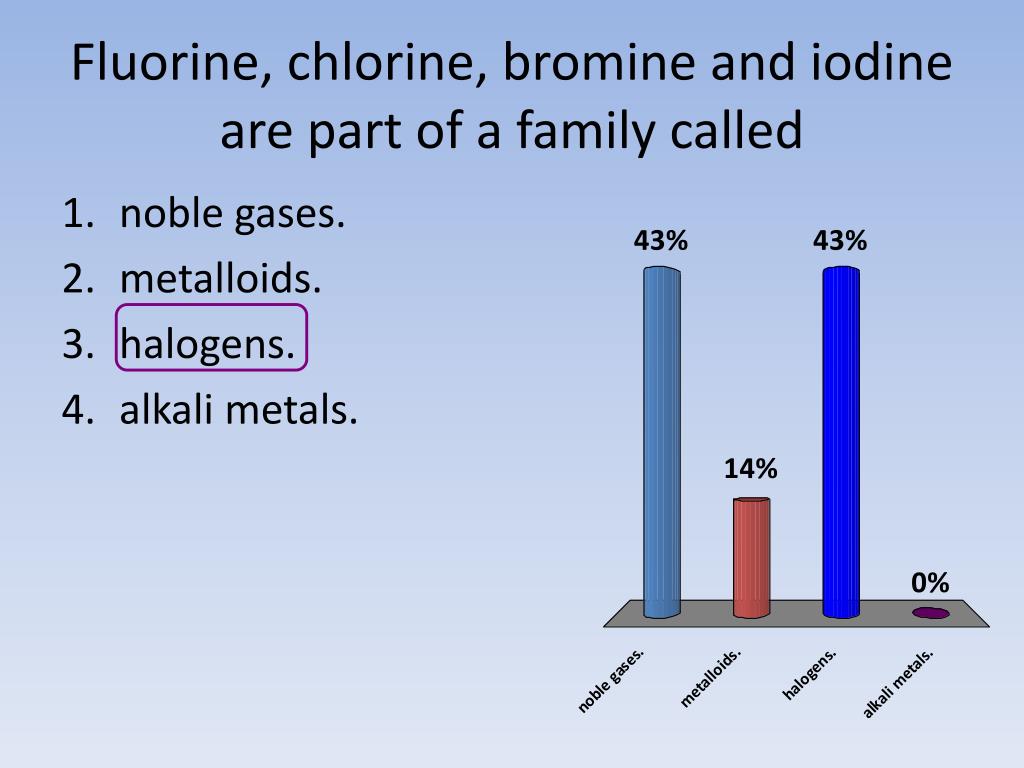
What Does Fluorine Chlorine And Bromine Have In Common . The three common group 7 elements are chlorine, bromine and iodine. fluorine is the most reactive element of all in group 7. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. You can see the trend in reactivity if you react the halogens with iron wool. the group 7 elements are also known as the halogens.
from www.slideserve.com
The three common group 7 elements are chlorine, bromine and iodine. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. You can see the trend in reactivity if you react the halogens with iron wool. fluorine is the most reactive element of all in group 7. the group 7 elements are also known as the halogens.
PPT Chapter 9 Elements & the Periodic Table PowerPoint Presentation
What Does Fluorine Chlorine And Bromine Have In Common The three common group 7 elements are chlorine, bromine and iodine. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. the group 7 elements are also known as the halogens. You can see the trend in reactivity if you react the halogens with iron wool. fluorine is the most reactive element of all in group 7. The three common group 7 elements are chlorine, bromine and iodine.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison What Does Fluorine Chlorine And Bromine Have In Common The three common group 7 elements are chlorine, bromine and iodine. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. the group 7 elements are also known as the halogens. You can see the trend in reactivity if you react the halogens with iron wool. fluorine is the most reactive. What Does Fluorine Chlorine And Bromine Have In Common.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library What Does Fluorine Chlorine And Bromine Have In Common The three common group 7 elements are chlorine, bromine and iodine. fluorine is the most reactive element of all in group 7. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. You can see the trend in reactivity if you react the halogens with iron wool. the group 7 elements. What Does Fluorine Chlorine And Bromine Have In Common.
From www.slideserve.com
PPT Chapter 9 Elements & the Periodic Table PowerPoint Presentation What Does Fluorine Chlorine And Bromine Have In Common fluorine is the most reactive element of all in group 7. You can see the trend in reactivity if you react the halogens with iron wool. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. fluorine generally oxidizes an element to its highest oxidation state,. What Does Fluorine Chlorine And Bromine Have In Common.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination What Does Fluorine Chlorine And Bromine Have In Common fluorine is the most reactive element of all in group 7. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. You can see the trend in reactivity if you react the halogens with iron wool. fluorine generally oxidizes an element to its highest oxidation state,. What Does Fluorine Chlorine And Bromine Have In Common.
From dxowxzkca.blob.core.windows.net
Fluorine Chlorine Bromine at Alphonse Sparks blog What Does Fluorine Chlorine And Bromine Have In Common fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. fluorine is the most reactive element of all in group 7. The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements are also known as the halogens. You can see the trend in reactivity if you. What Does Fluorine Chlorine And Bromine Have In Common.
From www.slideserve.com
PPT Chemistry UNIT 3 PowerPoint Presentation, free download ID5906142 What Does Fluorine Chlorine And Bromine Have In Common fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. the group 7 elements are also known as the halogens. fluorine is the most reactive element of all in group 7. You can see the trend in reactivity if you react the halogens with iron wool. The three common group 7. What Does Fluorine Chlorine And Bromine Have In Common.
From www.doheny.com
Bromine vs. Chlorine Everything You Need to Know What Does Fluorine Chlorine And Bromine Have In Common You can see the trend in reactivity if you react the halogens with iron wool. fluorine is the most reactive element of all in group 7. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. fluorine generally oxidizes an element to its highest oxidation state,. What Does Fluorine Chlorine And Bromine Have In Common.
From www.differencebetween.com
Difference Between Bromine and Chlorine Compare the Difference What Does Fluorine Chlorine And Bromine Have In Common fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. the group 7 elements are also known as the halogens. fluorine is the most reactive element of all in group 7. The three common group 7 elements are chlorine, bromine and iodine. You can see the trend in reactivity if you. What Does Fluorine Chlorine And Bromine Have In Common.
From www.slideshare.net
Lecture 6.1 The Periodic Table What Does Fluorine Chlorine And Bromine Have In Common fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. You can see the trend in reactivity if you react the halogens with iron wool. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. fluorine is the most reactive. What Does Fluorine Chlorine And Bromine Have In Common.
From slidetodoc.com
The periodic table Describe the chemical reactions that What Does Fluorine Chlorine And Bromine Have In Common fluorine is the most reactive element of all in group 7. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. the group 7 elements are also known as the halogens. You can see the trend in reactivity if you react the halogens with iron wool. The three common group 7. What Does Fluorine Chlorine And Bromine Have In Common.
From chemisfast.blogspot.com
Halogen family elementspropertiesperiodic tableoxyacids What Does Fluorine Chlorine And Bromine Have In Common fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. You can see the trend in reactivity if you react the halogens with iron wool. The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements are also known as the halogens. fluorine is the most reactive. What Does Fluorine Chlorine And Bromine Have In Common.
From dxowxzkca.blob.core.windows.net
Fluorine Chlorine Bromine at Alphonse Sparks blog What Does Fluorine Chlorine And Bromine Have In Common fluorine is the most reactive element of all in group 7. You can see the trend in reactivity if you react the halogens with iron wool. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements. What Does Fluorine Chlorine And Bromine Have In Common.
From www.slideserve.com
PPT What do we know about fluorine, chlorine and bromine? PowerPoint What Does Fluorine Chlorine And Bromine Have In Common fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. You can see the trend in reactivity if you react the halogens with iron wool. fluorine is the most reactive element of all in group 7. the group 7 elements are also known as the halogens. The three common group 7. What Does Fluorine Chlorine And Bromine Have In Common.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points What Does Fluorine Chlorine And Bromine Have In Common fluorine is the most reactive element of all in group 7. The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements are also known as the halogens. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. You can see the trend in reactivity if you. What Does Fluorine Chlorine And Bromine Have In Common.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms What Does Fluorine Chlorine And Bromine Have In Common fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. fluorine is the most reactive element of all in group 7. You can see the trend in reactivity if you. What Does Fluorine Chlorine And Bromine Have In Common.
From dxoltcoot.blob.core.windows.net
Bromine Chlorine And Fluorine Are Examples Of at Jessie Francis blog What Does Fluorine Chlorine And Bromine Have In Common You can see the trend in reactivity if you react the halogens with iron wool. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. fluorine is the most reactive element of all in group 7. the group 7 elements are also known as the halogens. The three common group 7. What Does Fluorine Chlorine And Bromine Have In Common.
From www.slideserve.com
PPT What do we know about fluorine, chlorine and bromine? PowerPoint What Does Fluorine Chlorine And Bromine Have In Common fluorine is the most reactive element of all in group 7. The three common group 7 elements are chlorine, bromine and iodine. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. the group 7 elements are also known as the halogens. You can see the trend in reactivity if you. What Does Fluorine Chlorine And Bromine Have In Common.
From www.alamy.com
Fluorine, Bromine and Chlorine Molecular Model of Atom. Vector What Does Fluorine Chlorine And Bromine Have In Common You can see the trend in reactivity if you react the halogens with iron wool. The three common group 7 elements are chlorine, bromine and iodine. fluorine is the most reactive element of all in group 7. the group 7 elements are also known as the halogens. fluorine generally oxidizes an element to its highest oxidation state,. What Does Fluorine Chlorine And Bromine Have In Common.
From www.askdifference.com
Fluoride vs. Chlorine — What’s the Difference? What Does Fluorine Chlorine And Bromine Have In Common fluorine is the most reactive element of all in group 7. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. You can see the trend in reactivity if you react the halogens with iron wool. The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements. What Does Fluorine Chlorine And Bromine Have In Common.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen What Does Fluorine Chlorine And Bromine Have In Common fluorine is the most reactive element of all in group 7. The three common group 7 elements are chlorine, bromine and iodine. You can see the trend in reactivity if you react the halogens with iron wool. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. the group 7 elements. What Does Fluorine Chlorine And Bromine Have In Common.
From dxomtvvmr.blob.core.windows.net
Iodine Bromine Chlorine And Fluorine at Herbert Brandt blog What Does Fluorine Chlorine And Bromine Have In Common fluorine is the most reactive element of all in group 7. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. You can see the trend in reactivity if you. What Does Fluorine Chlorine And Bromine Have In Common.
From www.youtube.com
THE HALOGENS FLUORINE, CHLORINE, BROMINE, IODINE AND ASTATINE. YouTube What Does Fluorine Chlorine And Bromine Have In Common You can see the trend in reactivity if you react the halogens with iron wool. The three common group 7 elements are chlorine, bromine and iodine. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. fluorine is the most reactive element of all in group 7. the group 7 elements. What Does Fluorine Chlorine And Bromine Have In Common.
From joelgordon.photoshelter.com
Chlorine Bromine Iodine Joel Gordon Photography What Does Fluorine Chlorine And Bromine Have In Common fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. The three common group 7 elements are chlorine, bromine and iodine. You can see the trend in reactivity if you react the halogens with iron wool. fluorine is the most reactive element of all in group 7. the group 7 elements. What Does Fluorine Chlorine And Bromine Have In Common.
From pediaa.com
Difference Between Bromine and Chlorine What Does Fluorine Chlorine And Bromine Have In Common You can see the trend in reactivity if you react the halogens with iron wool. the group 7 elements are also known as the halogens. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. The three common group 7 elements are chlorine, bromine and iodine. fluorine is the most reactive. What Does Fluorine Chlorine And Bromine Have In Common.
From www.youtube.com
Iodine, Chlorine, Bromine and Fluorine Relationship with Thyroid What Does Fluorine Chlorine And Bromine Have In Common The three common group 7 elements are chlorine, bromine and iodine. You can see the trend in reactivity if you react the halogens with iron wool. the group 7 elements are also known as the halogens. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. fluorine is the most reactive. What Does Fluorine Chlorine And Bromine Have In Common.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID1878282 What Does Fluorine Chlorine And Bromine Have In Common fluorine is the most reactive element of all in group 7. The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements are also known as the halogens. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. You can see the trend in reactivity if you. What Does Fluorine Chlorine And Bromine Have In Common.
From www.alamy.com
Chlorine atomic structure Cut Out Stock Images & Pictures Alamy What Does Fluorine Chlorine And Bromine Have In Common fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. the group 7 elements are also known as the halogens. You can see the trend in reactivity if you react the halogens with iron wool. The three common group 7 elements are chlorine, bromine and iodine. fluorine is the most reactive. What Does Fluorine Chlorine And Bromine Have In Common.
From www.youtube.com
How to draw Lewis dot structures Fluorine(F) Chlorine(Cl) Bromine(Br What Does Fluorine Chlorine And Bromine Have In Common The three common group 7 elements are chlorine, bromine and iodine. fluorine is the most reactive element of all in group 7. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. You can see the trend in reactivity if you react the halogens with iron wool. the group 7 elements. What Does Fluorine Chlorine And Bromine Have In Common.
From blog.chloramineconsulting.com
Chlorine vs. Bromine in Indoor Pools What Does Fluorine Chlorine And Bromine Have In Common fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. The three common group 7 elements are chlorine, bromine and iodine. fluorine is the most reactive element of all in group 7. You can see the trend in reactivity if you react the halogens with iron wool. the group 7 elements. What Does Fluorine Chlorine And Bromine Have In Common.
From slideplayer.com
Compounds Characteristics of Compounds Formation of Compounds Formulas What Does Fluorine Chlorine And Bromine Have In Common fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. The three common group 7 elements are chlorine, bromine and iodine. fluorine is the most reactive element of all in group 7. the group 7 elements are also known as the halogens. You can see the trend in reactivity if you. What Does Fluorine Chlorine And Bromine Have In Common.
From www.buzzle.com
Bromine Vs. Chlorine What Does Fluorine Chlorine And Bromine Have In Common fluorine is the most reactive element of all in group 7. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements are also known as the halogens. You can see the trend in reactivity if you. What Does Fluorine Chlorine And Bromine Have In Common.
From stock.adobe.com
Vecteur Stock Diatomic molecules diagram shows elements that exist as What Does Fluorine Chlorine And Bromine Have In Common the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. You can see the trend in reactivity if you react the halogens with iron wool. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. fluorine is the most reactive. What Does Fluorine Chlorine And Bromine Have In Common.
From www.differencebetween.com
Difference Between Bromine and Chlorine Compare the Difference What Does Fluorine Chlorine And Bromine Have In Common the group 7 elements are also known as the halogens. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. The three common group 7 elements are chlorine, bromine and iodine. fluorine is the most reactive element of all in group 7. You can see the trend in reactivity if you. What Does Fluorine Chlorine And Bromine Have In Common.
From nap.nationalacademies.org
Table of Isotopes of Fluorine, Chlorine, Bromine and Iodine The What Does Fluorine Chlorine And Bromine Have In Common fluorine is the most reactive element of all in group 7. You can see the trend in reactivity if you react the halogens with iron wool. The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements are also known as the halogens. fluorine generally oxidizes an element to its highest oxidation state,. What Does Fluorine Chlorine And Bromine Have In Common.
From studylib.net
Group 7 Elements What Does Fluorine Chlorine And Bromine Have In Common the group 7 elements are also known as the halogens. You can see the trend in reactivity if you react the halogens with iron wool. The three common group 7 elements are chlorine, bromine and iodine. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. fluorine is the most reactive. What Does Fluorine Chlorine And Bromine Have In Common.