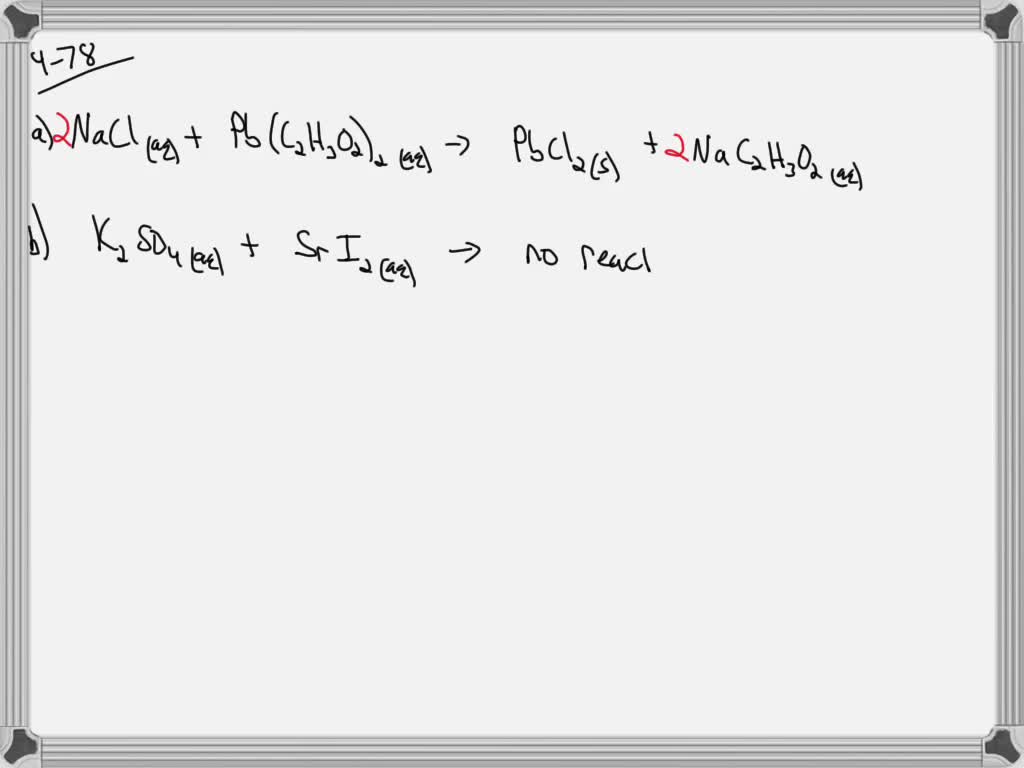Bromine And Potassium Iodide Chemical Equation . Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Chlorine gas, #cl_2#, reacts with potassium bromide, #kbr#, to form potassium chloride and bromine, #br_2#. Enter an equation of an ionic chemical equation and press the balance button. Because, bromine is more reactive than iodine and present in higher the 7th group. Balance a chemical equation when given the unbalanced equation. Chlorine + potassium iodide → potassium chloride + iodine. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Yes, bromine can react with potassium iodide. Explain the roles of subscripts and coefficients in chemical equations. Using a plastic pipette, add chlorine water, bromine water and iodine solution to the dimples of a spotting tile. The balanced equation will be calculated along with the. How do you write the. Chemical equation (h2o = h + o) 🛠️ A halide ion is an.
from www.numerade.com
Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Yes, bromine can react with potassium iodide. The balanced equation will be calculated along with the. Chemical equation (h2o = h + o) 🛠️ Balance a chemical equation when given the unbalanced equation. Using a plastic pipette, add chlorine water, bromine water and iodine solution to the dimples of a spotting tile. Chlorine + potassium iodide → potassium chloride + iodine. How do you write the. Because, bromine is more reactive than iodine and present in higher the 7th group. Explain the roles of subscripts and coefficients in chemical equations.
SOLVEDEnter a molecular equation for the precipitation reaction that
Bromine And Potassium Iodide Chemical Equation The balanced equation will be calculated along with the. Chlorine + potassium iodide → potassium chloride + iodine. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Yes, bromine can react with potassium iodide. Balance a chemical equation when given the unbalanced equation. Enter an equation of an ionic chemical equation and press the balance button. Using a plastic pipette, add chlorine water, bromine water and iodine solution to the dimples of a spotting tile. Explain the roles of subscripts and coefficients in chemical equations. Chlorine gas, #cl_2#, reacts with potassium bromide, #kbr#, to form potassium chloride and bromine, #br_2#. The balanced equation will be calculated along with the. How do you write the. Because, bromine is more reactive than iodine and present in higher the 7th group. Chemical equation (h2o = h + o) 🛠️ A halide ion is an. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Use the calculator below to balance chemical equations and determine the type of reaction (instructions).
From www.gauthmath.com
Solved Bromine reacts with potassium iodide in a single replacement Bromine And Potassium Iodide Chemical Equation A halide ion is an. How do you write the. Chlorine + potassium iodide → potassium chloride + iodine. Balance a chemical equation when given the unbalanced equation. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Yes, bromine can react with potassium iodide. Chlorine gas, #cl_2#,. Bromine And Potassium Iodide Chemical Equation.
From www.slideserve.com
PPT Double Replacement 1. Hydrogen sulfide is bubbled through a Bromine And Potassium Iodide Chemical Equation Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. Enter an equation of an ionic chemical equation and press the balance button. Yes, bromine can react with potassium iodide. A halide ion is an. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction. Bromine And Potassium Iodide Chemical Equation.
From www.dreamstime.com
Potassium Bromide Chemical Formula on Waterdrop Background Stock Bromine And Potassium Iodide Chemical Equation If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Chemical equation (h2o = h + o) 🛠️ A halide ion is an. Chlorine gas, #cl_2#, reacts with potassium bromide, #kbr#, to form potassium chloride and bromine, #br_2#. The balanced equation will be calculated along with the. Yes, bromine can react with potassium. Bromine And Potassium Iodide Chemical Equation.
From www.youtube.com
Net ionic equation Ammonium bromide plus barium hydroxide YouTube Bromine And Potassium Iodide Chemical Equation Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. How do you write the. Balance a chemical equation when given the unbalanced equation. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. A halide. Bromine And Potassium Iodide Chemical Equation.
From www.gauthmath.com
Solved Bromine reacts with potassium iodide in a single replacement Bromine And Potassium Iodide Chemical Equation Chlorine + potassium iodide → potassium chloride + iodine. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: How do you write the. The balanced equation will be calculated along with the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Cl 2 (aq) + 2ki. Bromine And Potassium Iodide Chemical Equation.
From www.coursehero.com
[Solved] Silver nitrate reacts with potassium iodide in aqueous Bromine And Potassium Iodide Chemical Equation Because, bromine is more reactive than iodine and present in higher the 7th group. Balance a chemical equation when given the unbalanced equation. Explain the roles of subscripts and coefficients in chemical equations. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Enter an equation of an ionic chemical equation and press. Bromine And Potassium Iodide Chemical Equation.
From www.toppr.com
Identify the type of reactions taking place in each of the following Bromine And Potassium Iodide Chemical Equation How do you write the. Chemical equation (h2o = h + o) 🛠️ Because, bromine is more reactive than iodine and present in higher the 7th group. Chlorine + potassium iodide → potassium chloride + iodine. Balance a chemical equation when given the unbalanced equation. Using a plastic pipette, add chlorine water, bromine water and iodine solution to the dimples. Bromine And Potassium Iodide Chemical Equation.
From www.numerade.com
SOLVED "solid potassium iodide into iodine gas and solid Bromine And Potassium Iodide Chemical Equation Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Chlorine gas, #cl_2#, reacts with potassium bromide, #kbr#, to form potassium chloride and bromine, #br_2#. The balanced equation will be calculated along with the. How do you write the. Use the calculator below to balance chemical equations and. Bromine And Potassium Iodide Chemical Equation.
From www.chegg.com
Solved Bromine liquid reacts with aqueous potassium iodide Bromine And Potassium Iodide Chemical Equation Enter an equation of an ionic chemical equation and press the balance button. Chemical equation (h2o = h + o) 🛠️ A halide ion is an. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Because, bromine is more reactive than iodine and present in higher the 7th group. Explain the roles. Bromine And Potassium Iodide Chemical Equation.
From www.youtube.com
Equation for KBr + H2O (Potassium bromide + Water) YouTube Bromine And Potassium Iodide Chemical Equation Enter an equation of an ionic chemical equation and press the balance button. Because, bromine is more reactive than iodine and present in higher the 7th group. Chemical equation (h2o = h + o) 🛠️ Chlorine + potassium iodide → potassium chloride + iodine. Explain the roles of subscripts and coefficients in chemical equations. A halide ion is an. If. Bromine And Potassium Iodide Chemical Equation.
From www.youtube.com
Bromine and potassium iodide YouTube Bromine And Potassium Iodide Chemical Equation Chlorine + potassium iodide → potassium chloride + iodine. Using a plastic pipette, add chlorine water, bromine water and iodine solution to the dimples of a spotting tile. A halide ion is an. Enter an equation of an ionic chemical equation and press the balance button. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. Bromine And Potassium Iodide Chemical Equation.
From www.chegg.com
Solved Solid potassium iodide into solid Bromine And Potassium Iodide Chemical Equation Chemical equation (h2o = h + o) 🛠️ Using a plastic pipette, add chlorine water, bromine water and iodine solution to the dimples of a spotting tile. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Chlorine gas, #cl_2#, reacts with potassium bromide, #kbr#, to form potassium. Bromine And Potassium Iodide Chemical Equation.
From www.alamy.com
Potassium supplement Cut Out Stock Images & Pictures Alamy Bromine And Potassium Iodide Chemical Equation Chlorine gas, #cl_2#, reacts with potassium bromide, #kbr#, to form potassium chloride and bromine, #br_2#. Because, bromine is more reactive than iodine and present in higher the 7th group. A halide ion is an. Explain the roles of subscripts and coefficients in chemical equations. How do you write the. If you add chlorine solution to colourless potassium bromide or potassium. Bromine And Potassium Iodide Chemical Equation.
From www.youtube.com
How to Balance KI + Br2 = KBr + I2 (Potassium iodide + Bromine gas Bromine And Potassium Iodide Chemical Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: How do you write the. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Chemical. Bromine And Potassium Iodide Chemical Equation.
From brainly.in
balance the equation potassium Bromide + barium iodide gives potassium Bromine And Potassium Iodide Chemical Equation How do you write the. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Using a plastic pipette, add chlorine water, bromine water and iodine solution to the dimples of a spotting tile. Chlorine gas, #cl_2#, reacts with potassium bromide, #kbr#, to form potassium chloride and bromine,. Bromine And Potassium Iodide Chemical Equation.
From www.youtube.com
Predict the Products Pb(NO3)2 + KI Lead (II) Nitrate + Potassium Bromine And Potassium Iodide Chemical Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Because, bromine is more reactive than iodine and present in higher the 7th group. Using a plastic pipette, add chlorine water, bromine water and iodine solution to the dimples of a spotting tile. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Bromine And Potassium Iodide Chemical Equation.
From www.gauthmath.com
Solved Bromine reacts with potassium iodide in a single replacement Bromine And Potassium Iodide Chemical Equation Using a plastic pipette, add chlorine water, bromine water and iodine solution to the dimples of a spotting tile. Enter an equation of an ionic chemical equation and press the balance button. Balance a chemical equation when given the unbalanced equation. Chemical equation (h2o = h + o) 🛠️ Use the calculator below to balance chemical equations and determine the. Bromine And Potassium Iodide Chemical Equation.
From www.gauthmath.com
Solved Bromine reacts with potassium iodide in a single replacement Bromine And Potassium Iodide Chemical Equation Explain the roles of subscripts and coefficients in chemical equations. Chemical equation (h2o = h + o) 🛠️ Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: The balanced equation. Bromine And Potassium Iodide Chemical Equation.
From www.numerade.com
SOLVED 'Reaction of sodium dichromate with potassium iodide in Bromine And Potassium Iodide Chemical Equation Chemical equation (h2o = h + o) 🛠️ The balanced equation will be calculated along with the. Balance a chemical equation when given the unbalanced equation. A halide ion is an. Chlorine gas, #cl_2#, reacts with potassium bromide, #kbr#, to form potassium chloride and bromine, #br_2#. Enter an equation of an ionic chemical equation and press the balance button. Using. Bromine And Potassium Iodide Chemical Equation.
From www.numerade.com
SOLVED Solid potassium iodide into iodine gas and solid Bromine And Potassium Iodide Chemical Equation A halide ion is an. Because, bromine is more reactive than iodine and present in higher the 7th group. Explain the roles of subscripts and coefficients in chemical equations. The balanced equation will be calculated along with the. Chemical equation (h2o = h + o) 🛠️ Balance a chemical equation when given the unbalanced equation. Cl 2 (aq) + 2ki. Bromine And Potassium Iodide Chemical Equation.
From brainly.in
Reaction between sodium iodide and bromine water Brainly.in Bromine And Potassium Iodide Chemical Equation Enter an equation of an ionic chemical equation and press the balance button. Chlorine + potassium iodide → potassium chloride + iodine. How do you write the. Chlorine gas, #cl_2#, reacts with potassium bromide, #kbr#, to form potassium chloride and bromine, #br_2#. Yes, bromine can react with potassium iodide. If you add chlorine solution to colourless potassium bromide or potassium. Bromine And Potassium Iodide Chemical Equation.
From www.youtube.com
How to Write the Net Ionic Equation for KI + Cl2 = KCl + I2 YouTube Bromine And Potassium Iodide Chemical Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Because, bromine is more reactive than iodine and present in higher the 7th group. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Yes, bromine can react with potassium iodide. Chlorine +. Bromine And Potassium Iodide Chemical Equation.
From www.thesciencehive.co.uk
Group7halogensanswers — the science sauce Bromine And Potassium Iodide Chemical Equation Using a plastic pipette, add chlorine water, bromine water and iodine solution to the dimples of a spotting tile. Enter an equation of an ionic chemical equation and press the balance button. Chlorine + potassium iodide → potassium chloride + iodine. The balanced equation will be calculated along with the. A halide ion is an. Because, bromine is more reactive. Bromine And Potassium Iodide Chemical Equation.
From www.youtube.com
How to Write the Net Ionic Equation for KI + Br2 = KBr + I2 YouTube Bromine And Potassium Iodide Chemical Equation Chemical equation (h2o = h + o) 🛠️ Balance a chemical equation when given the unbalanced equation. Chlorine + potassium iodide → potassium chloride + iodine. How do you write the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). If you add chlorine solution to colourless potassium bromide or potassium iodide solution a. Bromine And Potassium Iodide Chemical Equation.
From www.numerade.com
SOLVEDEnter a molecular equation for the precipitation reaction that Bromine And Potassium Iodide Chemical Equation Balance a chemical equation when given the unbalanced equation. Chlorine gas, #cl_2#, reacts with potassium bromide, #kbr#, to form potassium chloride and bromine, #br_2#. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Because, bromine is more reactive than iodine and present in higher the 7th group. A halide ion is an. Chlorine +. Bromine And Potassium Iodide Chemical Equation.
From brendajbrown.blogspot.com
View What Is Potassium Bromide Made Of Pictures Bromine And Potassium Iodide Chemical Equation How do you write the. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: A halide ion is an. Yes, bromine can react with potassium iodide. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Because, bromine is more. Bromine And Potassium Iodide Chemical Equation.
From slideplayer.com
Chemsheets AS006 (Electron arrangement) ppt download Bromine And Potassium Iodide Chemical Equation Balance a chemical equation when given the unbalanced equation. Chlorine gas, #cl_2#, reacts with potassium bromide, #kbr#, to form potassium chloride and bromine, #br_2#. Because, bromine is more reactive than iodine and present in higher the 7th group. How do you write the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). If you. Bromine And Potassium Iodide Chemical Equation.
From www.numerade.com
SOLVED write the balanced equation and identify the type of reaction Bromine And Potassium Iodide Chemical Equation Balance a chemical equation when given the unbalanced equation. Chlorine gas, #cl_2#, reacts with potassium bromide, #kbr#, to form potassium chloride and bromine, #br_2#. Enter an equation of an ionic chemical equation and press the balance button. Chemical equation (h2o = h + o) 🛠️ A halide ion is an. Explain the roles of subscripts and coefficients in chemical equations.. Bromine And Potassium Iodide Chemical Equation.
From www.numerade.com
SOLVEDGaseous chlorine will displace bromide ion from an aqueous Bromine And Potassium Iodide Chemical Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Because, bromine is more reactive than iodine and present in higher the 7th group. Yes, bromine can react with potassium iodide. How do you write the. Chlorine + potassium iodide → potassium chloride + iodine. Chlorine gas, #cl_2#, reacts with potassium bromide, #kbr#, to form. Bromine And Potassium Iodide Chemical Equation.
From slideplayer.com
Reaction Stoichiometry ppt download Bromine And Potassium Iodide Chemical Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: A halide ion is an. How do you write the. Using a plastic pipette, add chlorine water, bromine water and iodine solution to the dimples of a spotting. Bromine And Potassium Iodide Chemical Equation.
From www.numerade.com
Chlorine gas is bubbled through an aqueous solution of potassium iodide Bromine And Potassium Iodide Chemical Equation Yes, bromine can react with potassium iodide. Balance a chemical equation when given the unbalanced equation. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Because, bromine is more reactive than iodine and present in higher the 7th group. A halide ion is an. If you add. Bromine And Potassium Iodide Chemical Equation.
From www.numerade.com
SOLVED Write a balanced molecular equation for a reaction between Bromine And Potassium Iodide Chemical Equation Chemical equation (h2o = h + o) 🛠️ The balanced equation will be calculated along with the. How do you write the. Chlorine + potassium iodide → potassium chloride + iodine. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Use the calculator below to balance chemical. Bromine And Potassium Iodide Chemical Equation.
From www.youtube.com
Bromine and potassium iodide YouTube Bromine And Potassium Iodide Chemical Equation Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Enter an equation of an ionic chemical equation and press the balance button. Because, bromine is more reactive than iodine and present in higher the 7th group. If you add chlorine solution to colourless potassium bromide or potassium. Bromine And Potassium Iodide Chemical Equation.
From www.toppr.com
Write the balanced chemical equation for the following and identify the Bromine And Potassium Iodide Chemical Equation Chlorine + potassium iodide → potassium chloride + iodine. Enter an equation of an ionic chemical equation and press the balance button. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Yes, bromine can react with potassium iodide. Balance a chemical equation when given the unbalanced equation. How do you write the.. Bromine And Potassium Iodide Chemical Equation.
From www.researchgate.net
A.) Chemical reaction between hydrogen peroxide and potassium iodide Bromine And Potassium Iodide Chemical Equation Enter an equation of an ionic chemical equation and press the balance button. Chlorine + potassium iodide → potassium chloride + iodine. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. How do you write the. Balance a chemical equation when given the unbalanced equation. Chlorine gas,. Bromine And Potassium Iodide Chemical Equation.