Bomb Calorimeter Bbc Bitesize . A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. An improvement could be to use a bomb calorimeter: A simple calorimeter can be made from a polystyrene drinking cup, a. A variety of fuels undergo combustion, and the products made can be identified. In essence, a bomb calorimeter measures the heat of combustion of a sample at constant volume which is then used to determine calorific value,. Calorimetry is the measurement enthalpy changes in chemical reactions. Have you ever wondered why some foods give you more energy than others? Sample is burned in a sealed chamber to reduce heat loss to the environment. Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid or solid materials. The fire triangle identifies the three. How can we measure the heat of reaction.
from www.chegg.com
The fire triangle identifies the three. Sample is burned in a sealed chamber to reduce heat loss to the environment. In essence, a bomb calorimeter measures the heat of combustion of a sample at constant volume which is then used to determine calorific value,. Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid or solid materials. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A simple calorimeter can be made from a polystyrene drinking cup, a. How can we measure the heat of reaction. Have you ever wondered why some foods give you more energy than others? An improvement could be to use a bomb calorimeter: Calorimetry is the measurement enthalpy changes in chemical reactions.
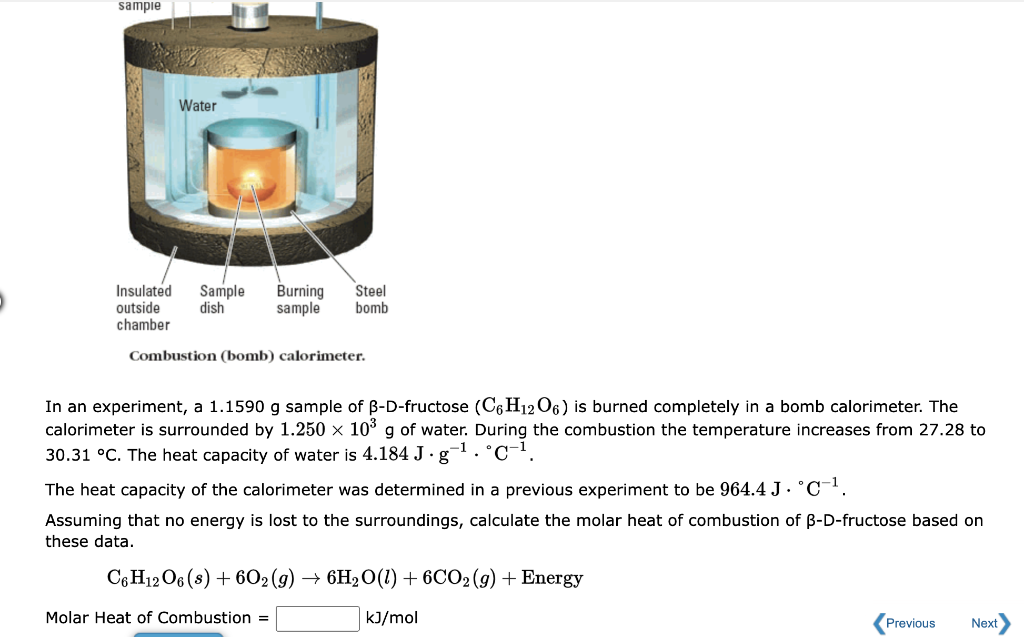
Solved Combustion (bomb) calorimeter. In an experiment, a
Bomb Calorimeter Bbc Bitesize Calorimetry is the measurement enthalpy changes in chemical reactions. In essence, a bomb calorimeter measures the heat of combustion of a sample at constant volume which is then used to determine calorific value,. Have you ever wondered why some foods give you more energy than others? Sample is burned in a sealed chamber to reduce heat loss to the environment. The fire triangle identifies the three. How can we measure the heat of reaction. A simple calorimeter can be made from a polystyrene drinking cup, a. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Calorimetry is the measurement enthalpy changes in chemical reactions. An improvement could be to use a bomb calorimeter: A variety of fuels undergo combustion, and the products made can be identified. Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid or solid materials.
From www.chegg.com
Solved Thermometer A bomb calorimeter, or constant volume Bomb Calorimeter Bbc Bitesize A variety of fuels undergo combustion, and the products made can be identified. Have you ever wondered why some foods give you more energy than others? A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The fire triangle identifies the. Bomb Calorimeter Bbc Bitesize.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Bbc Bitesize In essence, a bomb calorimeter measures the heat of combustion of a sample at constant volume which is then used to determine calorific value,. A simple calorimeter can be made from a polystyrene drinking cup, a. The fire triangle identifies the three. Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid. Bomb Calorimeter Bbc Bitesize.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Bomb Calorimeter Bbc Bitesize A variety of fuels undergo combustion, and the products made can be identified. Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid or solid materials. Calorimetry is the measurement enthalpy changes in chemical reactions. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned. Bomb Calorimeter Bbc Bitesize.
From ar.inspiredpencil.com
Bomb Calorimeter Setup Bomb Calorimeter Bbc Bitesize Have you ever wondered why some foods give you more energy than others? Sample is burned in a sealed chamber to reduce heat loss to the environment. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A variety of fuels. Bomb Calorimeter Bbc Bitesize.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter Bbc Bitesize The fire triangle identifies the three. An improvement could be to use a bomb calorimeter: A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion. Bomb Calorimeter Bbc Bitesize.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Bomb Calorimeter Bbc Bitesize How can we measure the heat of reaction. A simple calorimeter can be made from a polystyrene drinking cup, a. Calorimetry is the measurement enthalpy changes in chemical reactions. An improvement could be to use a bomb calorimeter: Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid or solid materials. Have. Bomb Calorimeter Bbc Bitesize.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Bomb Calorimeter Bbc Bitesize An improvement could be to use a bomb calorimeter: A variety of fuels undergo combustion, and the products made can be identified. Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid or solid materials. A simple calorimeter can be made from a polystyrene drinking cup, a. How can we measure the. Bomb Calorimeter Bbc Bitesize.
From byjus.com
What is bomb calorimeter? Bomb Calorimeter Bbc Bitesize A simple calorimeter can be made from a polystyrene drinking cup, a. Have you ever wondered why some foods give you more energy than others? The fire triangle identifies the three. Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid or solid materials. A variety of fuels undergo combustion, and the. Bomb Calorimeter Bbc Bitesize.
From www.chegg.com
Solved A bomb calorimeter, or a constant volume calorimeter, Bomb Calorimeter Bbc Bitesize A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. In essence, a bomb calorimeter measures the heat of combustion of a sample at constant volume which is then used to determine calorific value,. Bomb calorimetry is a device used for. Bomb Calorimeter Bbc Bitesize.
From www.researchgate.net
Schematic sketch of a bomb calorimeter Download Scientific Diagram Bomb Calorimeter Bbc Bitesize Have you ever wondered why some foods give you more energy than others? The fire triangle identifies the three. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Sample is burned in a sealed chamber to reduce heat loss to. Bomb Calorimeter Bbc Bitesize.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Bomb Calorimeter Bbc Bitesize An improvement could be to use a bomb calorimeter: The fire triangle identifies the three. Calorimetry is the measurement enthalpy changes in chemical reactions. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Sample is burned in a sealed chamber. Bomb Calorimeter Bbc Bitesize.
From saylordotorg.github.io
Calorimetry Bomb Calorimeter Bbc Bitesize A simple calorimeter can be made from a polystyrene drinking cup, a. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Calorimetry is the measurement enthalpy changes in chemical reactions. In essence, a bomb calorimeter measures the heat of combustion. Bomb Calorimeter Bbc Bitesize.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Bomb Calorimeter Bbc Bitesize A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A simple calorimeter can be made from a polystyrene drinking cup, a. Sample is burned in a sealed chamber to reduce heat loss to the environment. Bomb calorimetry is a device. Bomb Calorimeter Bbc Bitesize.
From foodtechnews.in
What Is Bomb Calorimeter🤔 Measurement of Energy Content in food Food Bomb Calorimeter Bbc Bitesize Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid or solid materials. How can we measure the heat of reaction. The fire triangle identifies the three. A variety of fuels undergo combustion, and the products made can be identified. Calorimetry is the measurement enthalpy changes in chemical reactions. An improvement could. Bomb Calorimeter Bbc Bitesize.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Bbc Bitesize How can we measure the heat of reaction. A variety of fuels undergo combustion, and the products made can be identified. Have you ever wondered why some foods give you more energy than others? Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid or solid materials. A simple calorimeter can be. Bomb Calorimeter Bbc Bitesize.
From ar.inspiredpencil.com
Bomb Calorimeter Setup Bomb Calorimeter Bbc Bitesize A simple calorimeter can be made from a polystyrene drinking cup, a. A variety of fuels undergo combustion, and the products made can be identified. The fire triangle identifies the three. An improvement could be to use a bomb calorimeter: Calorimetry is the measurement enthalpy changes in chemical reactions. A bomb calorimeter is used to measure, under controlled conditions, the. Bomb Calorimeter Bbc Bitesize.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] Bomb Calorimeter Bbc Bitesize Have you ever wondered why some foods give you more energy than others? Calorimetry is the measurement enthalpy changes in chemical reactions. A simple calorimeter can be made from a polystyrene drinking cup, a. A variety of fuels undergo combustion, and the products made can be identified. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted. Bomb Calorimeter Bbc Bitesize.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Bbc Bitesize An improvement could be to use a bomb calorimeter: In essence, a bomb calorimeter measures the heat of combustion of a sample at constant volume which is then used to determine calorific value,. Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid or solid materials. A simple calorimeter can be made. Bomb Calorimeter Bbc Bitesize.
From animalia-life.club
Simple Bomb Calorimeter Bomb Calorimeter Bbc Bitesize An improvement could be to use a bomb calorimeter: How can we measure the heat of reaction. In essence, a bomb calorimeter measures the heat of combustion of a sample at constant volume which is then used to determine calorific value,. A variety of fuels undergo combustion, and the products made can be identified. Have you ever wondered why some. Bomb Calorimeter Bbc Bitesize.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Bomb Calorimeter Bbc Bitesize Calorimetry is the measurement enthalpy changes in chemical reactions. An improvement could be to use a bomb calorimeter: In essence, a bomb calorimeter measures the heat of combustion of a sample at constant volume which is then used to determine calorific value,. A simple calorimeter can be made from a polystyrene drinking cup, a. Bomb calorimetry is a device used. Bomb Calorimeter Bbc Bitesize.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Bomb Calorimeter Bbc Bitesize A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. An improvement could be to use a bomb calorimeter: In essence, a bomb calorimeter measures the heat of combustion of a sample at constant volume which is then used to determine. Bomb Calorimeter Bbc Bitesize.
From pubs.sciepub.com
Figure 1. Diagram of Bomb Calorimeter used in this Study Measuring Bomb Calorimeter Bbc Bitesize Have you ever wondered why some foods give you more energy than others? A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A simple calorimeter can be made from a polystyrene drinking cup, a. A variety of fuels undergo combustion,. Bomb Calorimeter Bbc Bitesize.
From www.youtube.com
Bomb Calorimeter Definition, Construction, Working & Uses YouTube Bomb Calorimeter Bbc Bitesize A simple calorimeter can be made from a polystyrene drinking cup, a. How can we measure the heat of reaction. The fire triangle identifies the three. Calorimetry is the measurement enthalpy changes in chemical reactions. An improvement could be to use a bomb calorimeter: In essence, a bomb calorimeter measures the heat of combustion of a sample at constant volume. Bomb Calorimeter Bbc Bitesize.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter Bbc Bitesize How can we measure the heat of reaction. A simple calorimeter can be made from a polystyrene drinking cup, a. Have you ever wondered why some foods give you more energy than others? Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid or solid materials. An improvement could be to use. Bomb Calorimeter Bbc Bitesize.
From ar.inspiredpencil.com
Bomb Calorimeter Setup Bomb Calorimeter Bbc Bitesize An improvement could be to use a bomb calorimeter: A simple calorimeter can be made from a polystyrene drinking cup, a. The fire triangle identifies the three. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Bomb calorimetry is a. Bomb Calorimeter Bbc Bitesize.
From gamma.app
Bomb Calorimeter A Comprehensive Guide Bomb Calorimeter Bbc Bitesize An improvement could be to use a bomb calorimeter: A simple calorimeter can be made from a polystyrene drinking cup, a. Have you ever wondered why some foods give you more energy than others? Calorimetry is the measurement enthalpy changes in chemical reactions. How can we measure the heat of reaction. A bomb calorimeter is used to measure, under controlled. Bomb Calorimeter Bbc Bitesize.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Bomb Calorimeter Bbc Bitesize A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Have you ever wondered why some foods give you more energy than others? A variety of fuels undergo combustion, and the products made can be identified. How can we measure the. Bomb Calorimeter Bbc Bitesize.
From www.labster.com
Calorimetry Using a bomb calorimeter Virtual Lab Bomb Calorimeter Bbc Bitesize A variety of fuels undergo combustion, and the products made can be identified. In essence, a bomb calorimeter measures the heat of combustion of a sample at constant volume which is then used to determine calorific value,. The fire triangle identifies the three. A simple calorimeter can be made from a polystyrene drinking cup, a. Calorimetry is the measurement enthalpy. Bomb Calorimeter Bbc Bitesize.
From wps.prenhall.com
Media Portfolio Bomb Calorimeter Bbc Bitesize Calorimetry is the measurement enthalpy changes in chemical reactions. How can we measure the heat of reaction. In essence, a bomb calorimeter measures the heat of combustion of a sample at constant volume which is then used to determine calorific value,. An improvement could be to use a bomb calorimeter: A variety of fuels undergo combustion, and the products made. Bomb Calorimeter Bbc Bitesize.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter Bbc Bitesize A simple calorimeter can be made from a polystyrene drinking cup, a. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. How can we measure the heat of reaction. Have you ever wondered why some foods give you more energy. Bomb Calorimeter Bbc Bitesize.
From slideplayer.com
Lecture 2 Calculating the change of enthalpy and reaction enthalpy Bomb Calorimeter Bbc Bitesize Calorimetry is the measurement enthalpy changes in chemical reactions. A variety of fuels undergo combustion, and the products made can be identified. A simple calorimeter can be made from a polystyrene drinking cup, a. Have you ever wondered why some foods give you more energy than others? An improvement could be to use a bomb calorimeter: The fire triangle identifies. Bomb Calorimeter Bbc Bitesize.
From ar.inspiredpencil.com
Bomb Calorimeter Setup Bomb Calorimeter Bbc Bitesize How can we measure the heat of reaction. Sample is burned in a sealed chamber to reduce heat loss to the environment. Calorimetry is the measurement enthalpy changes in chemical reactions. The fire triangle identifies the three. A variety of fuels undergo combustion, and the products made can be identified. A bomb calorimeter is used to measure, under controlled conditions,. Bomb Calorimeter Bbc Bitesize.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Bbc Bitesize A simple calorimeter can be made from a polystyrene drinking cup, a. Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid or solid materials. Have you ever wondered why some foods give you more energy than others? How can we measure the heat of reaction. Calorimetry is the measurement enthalpy changes. Bomb Calorimeter Bbc Bitesize.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID9276632 Bomb Calorimeter Bbc Bitesize Have you ever wondered why some foods give you more energy than others? A simple calorimeter can be made from a polystyrene drinking cup, a. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Sample is burned in a sealed. Bomb Calorimeter Bbc Bitesize.
From www.chegg.com
Solved Combustion (bomb) calorimeter. In an experiment, a Bomb Calorimeter Bbc Bitesize Bomb calorimetry is a device used for measuring the amount of heat generated from the combustion of liquid or solid materials. An improvement could be to use a bomb calorimeter: In essence, a bomb calorimeter measures the heat of combustion of a sample at constant volume which is then used to determine calorific value,. A simple calorimeter can be made. Bomb Calorimeter Bbc Bitesize.