Iodometric Titration End Point . The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. One drop of 0,05 m iodine solution. Starch solution is used for end point detection in iodometric titration. A solution of iodine in aqueous iodide has an intense yellow to brown colour. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. To prepare starch indicator solution, add 1 gram of starch (either. How can the end point of an iodometric titration be determined? As has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. Add 50 ml of deionized.
from www.slideshare.net
A solution of iodine in aqueous iodide has an intense yellow to brown colour. Add 50 ml of deionized. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. One drop of 0,05 m iodine solution. A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. How can the end point of an iodometric titration be determined? To prepare starch indicator solution, add 1 gram of starch (either. As has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. Starch solution is used for end point detection in iodometric titration. The endpoint of the titration is located with the starch indicator solution that was also prepared earlier.
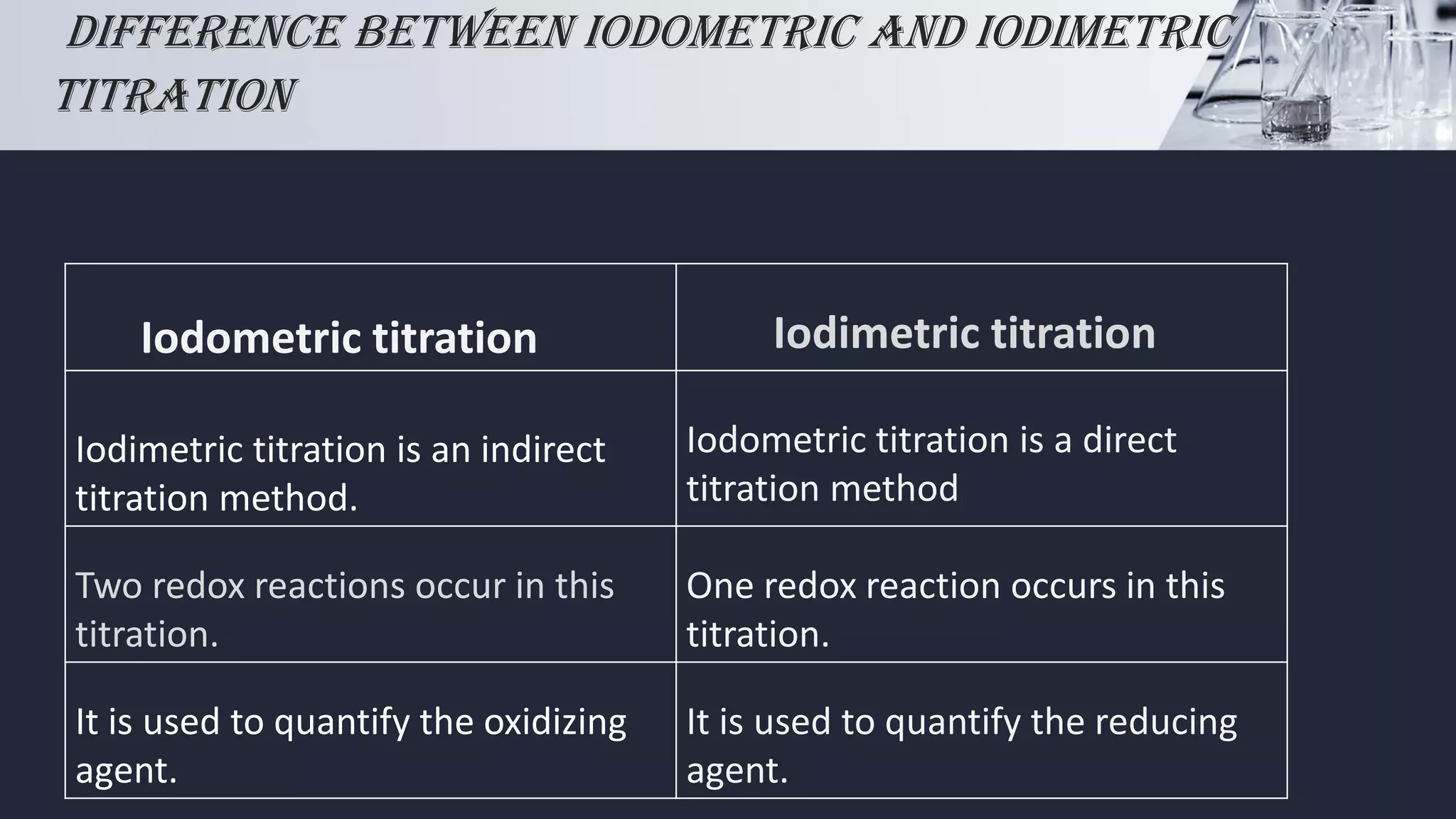
Iodometric and iodimetric Titration PPT
Iodometric Titration End Point Add 50 ml of deionized. Add 50 ml of deionized. One drop of 0,05 m iodine solution. Starch solution is used for end point detection in iodometric titration. A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. How can the end point of an iodometric titration be determined? To prepare starch indicator solution, add 1 gram of starch (either. As has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. A solution of iodine in aqueous iodide has an intense yellow to brown colour.
From byjus.com
22. Which of the following can give Iodometric titration A)Fe+3 B)Cu+2 Iodometric Titration End Point Add 50 ml of deionized. Starch solution is used for end point detection in iodometric titration. How can the end point of an iodometric titration be determined? A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. To prepare starch indicator solution, add 1 gram of starch (either. As has. Iodometric Titration End Point.
From themasterchemistry.com
Iodometric Titration Principle, Example, Advantages Iodometric Titration End Point Add 50 ml of deionized. A solution of iodine in aqueous iodide has an intense yellow to brown colour. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. Starch solution is used for end. Iodometric Titration End Point.
From www.animalia-life.club
Endpoint Titration Iodometric Titration End Point Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Starch solution is used for end point detection in iodometric titration. To prepare starch indicator solution, add 1 gram of starch (either. How can the end point of an iodometric titration be determined? A starch solution is used as an indicator in. Iodometric Titration End Point.
From www.microlit.com
An Advanced Guide to Titration Microlit Iodometric Titration End Point Starch solution is used for end point detection in iodometric titration. A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. Add 50 ml of deionized. A solution of iodine in aqueous iodide has an intense yellow to brown colour. Thanks to its relatively low, ph independent redox potential, and. Iodometric Titration End Point.
From gbu-taganskij.ru
Iodometric Titration Principle, Example, Advantages, 44 OFF Iodometric Titration End Point As has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. To prepare starch indicator solution, add 1 gram of starch (either. Add 50 ml of deionized. Starch solution is used for end point detection in iodometric titration. Thanks to its relatively low, ph independent redox potential,. Iodometric Titration End Point.
From gbu-presnenskij.ru
Iodometric Titration Principle, Example, Advantages, 51 OFF Iodometric Titration End Point A solution of iodine in aqueous iodide has an intense yellow to brown colour. To prepare starch indicator solution, add 1 gram of starch (either. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. The endpoint of the titration is located with the starch indicator solution that was also prepared earlier.. Iodometric Titration End Point.
From questions-in.kunduz.com
IN IODOMETRIC TITRATION hydrogen peroxid... Physical Chemistry Iodometric Titration End Point As has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. How can the end point of an iodometric titration be determined? Starch solution is used for end point detection in iodometric titration. One drop of 0,05 m iodine solution. A starch solution is used as an. Iodometric Titration End Point.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Iodometric Titration End Point A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. A solution of iodine in aqueous iodide has an intense yellow to brown colour. To prepare starch indicator solution, add 1 gram of starch (either. How can the end point of an iodometric titration be determined? The endpoint of the. Iodometric Titration End Point.
From www.animalia-life.club
Endpoint Titration Iodometric Titration End Point A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. One drop of 0,05 m iodine solution. To prepare starch indicator solution, add 1 gram of starch (either. A solution of iodine in. Iodometric Titration End Point.
From www.numerade.com
SOLVED iodometric titration of vitamin C 5g KI and 0.285g KIO3 are Iodometric Titration End Point How can the end point of an iodometric titration be determined? A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. A solution of iodine in aqueous iodide has an intense yellow to brown colour. The endpoint of the titration is located with the starch indicator solution that was also. Iodometric Titration End Point.
From www.researchgate.net
(a) Iodometric titration to determine stoichiometry of ACTU and BrO 3 Iodometric Titration End Point One drop of 0,05 m iodine solution. As has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. A solution of iodine in aqueous iodide has an intense yellow to brown colour. A starch solution is used as an indicator in an iodometric titration because it can. Iodometric Titration End Point.
From www.youtube.com
End Point Determination by Potentiometry Titrations Pharmaceutical Iodometric Titration End Point A solution of iodine in aqueous iodide has an intense yellow to brown colour. A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. Add 50 ml of deionized. One drop of 0,05 m iodine solution. To prepare starch indicator solution, add 1 gram of starch (either. Thanks to its. Iodometric Titration End Point.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Iodometric Titration End Point Starch solution is used for end point detection in iodometric titration. As has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. How can the end point of an iodometric titration be determined? To prepare starch indicator solution, add 1 gram of starch (either. Thanks to its. Iodometric Titration End Point.
From pixels.com
End Point Of An Iodine Titration. 3 Of 5. Photograph by Martyn F Iodometric Titration End Point The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. Add 50 ml of deionized. To prepare starch indicator solution, add 1 gram of starch (either. How can the end point of an iodometric titration be determined? Starch solution is used for end point detection in iodometric titration. A solution of iodine in. Iodometric Titration End Point.
From ar.inspiredpencil.com
Endpoint Titration Iodometric Titration End Point Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. As has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. Starch solution is used for end point detection in iodometric titration. A starch solution is used as an. Iodometric Titration End Point.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Iodometric Titration End Point As has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. A solution of iodine in aqueous iodide has an intense yellow to brown colour. To prepare starch indicator solution,. Iodometric Titration End Point.
From themasterchemistry.com
Iodometric Titration Principle, Example, Advantages Iodometric Titration End Point How can the end point of an iodometric titration be determined? As has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. The endpoint of the titration. Iodometric Titration End Point.
From www.academia.edu
(PDF) Voltammetric Iodometric Titration of Ascorbic Acid with DeadStop Iodometric Titration End Point Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. A solution of iodine in aqueous iodide has an intense yellow to brown colour. A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. The endpoint of the titration is located with. Iodometric Titration End Point.
From mymumbaipost.in
Explore 10 Key Difference between Iodometry and Iodimetry Mymumbaipost Iodometric Titration End Point A solution of iodine in aqueous iodide has an intense yellow to brown colour. The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. How can the end point of an iodometric titration. Iodometric Titration End Point.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Iodometric Titration End Point The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. A solution of iodine in aqueous iodide has an intense yellow to brown colour. One drop of 0,05 m iodine solution. How can the end point of an iodometric titration be determined? Thanks to its relatively low, ph independent redox potential, and reversibility. Iodometric Titration End Point.
From www.numerade.com
SOLVED Iodometric Titrations Oxidant (analyte) converts iodide to Iodometric Titration End Point A solution of iodine in aqueous iodide has an intense yellow to brown colour. Add 50 ml of deionized. To prepare starch indicator solution, add 1 gram of starch (either. How can the end point of an iodometric titration be determined? A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that. Iodometric Titration End Point.
From www.toppr.com
The end point of iodometric titrations is detected by adding starch Iodometric Titration End Point The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. Add 50 ml of deionized. One drop of 0,05 m iodine solution. To prepare starch indicator solution, add 1 gram of starch (either. A solution of iodine in aqueous iodide has an intense yellow to brown colour. How can the end point of. Iodometric Titration End Point.
From www.researchgate.net
(a) Iodometric titration to determine stoichiometry of ACTU and BrO 3 Iodometric Titration End Point Starch solution is used for end point detection in iodometric titration. Add 50 ml of deionized. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. A starch solution is used as an indicator in. Iodometric Titration End Point.
From www.studypool.com
SOLUTION Dichromate and iodometric titrations chemistry lab report Iodometric Titration End Point Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. One drop of 0,05 m iodine solution. Starch solution is used for end point detection in iodometric titration. How can the end point of an iodometric titration be determined? As has been mentioned above, the endpoint in a titration of iodine with. Iodometric Titration End Point.
From www.youtube.com
Redox Titration (Iodometric) YouTube Iodometric Titration End Point One drop of 0,05 m iodine solution. Starch solution is used for end point detection in iodometric titration. As has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be.. Iodometric Titration End Point.
From www.chemedx.org
Determination of Vitamin C in a Produce Protector Iodometric Method Iodometric Titration End Point One drop of 0,05 m iodine solution. Starch solution is used for end point detection in iodometric titration. Add 50 ml of deionized. To prepare starch indicator solution, add 1 gram of starch (either. The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. How can the end point of an iodometric titration. Iodometric Titration End Point.
From www.researchgate.net
The scheme of the equipment used for iodometric titration 1 , 2 Iodometric Titration End Point Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. One drop of 0,05 m iodine solution. Add 50 ml of deionized. The endpoint of the titration is located with the starch. Iodometric Titration End Point.
From www.youtube.com
CHEMY101 Experiment 8 Iodometric titration YouTube Iodometric Titration End Point Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Add 50 ml of deionized. To prepare starch indicator solution, add 1 gram of starch (either. As has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. The endpoint. Iodometric Titration End Point.
From www.slideshare.net
Iodometric and iodimetric Titration PPT Iodometric Titration End Point Add 50 ml of deionized. One drop of 0,05 m iodine solution. To prepare starch indicator solution, add 1 gram of starch (either. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. How can. Iodometric Titration End Point.
From www.youtube.com
Estimation of Cu2+ ions by Iodometric Titration Method Iodometric Iodometric Titration End Point The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. A starch solution is used as an indicator in an iodometric titration because it can absorb the i2 that is. How can the end point of an iodometric titration be determined? Starch solution is used for end point detection in iodometric titration. Thanks. Iodometric Titration End Point.
From www.alamy.com
Finding the end point of an iodine titration. 1 of 5. At the start of Iodometric Titration End Point The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. A solution of iodine in aqueous iodide has an intense yellow to brown colour. As has been mentioned above, the endpoint in a titration of. Iodometric Titration End Point.
From www.doubtnut.com
In Iodometric titration which indicator is used to detect end point of Iodometric Titration End Point The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. Starch solution is used for end point detection in iodometric titration. As has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. To prepare starch indicator solution, add 1 gram. Iodometric Titration End Point.
From www.researchgate.net
Iodometric titration for quantitative characterization of NCl content Iodometric Titration End Point Add 50 ml of deionized. A solution of iodine in aqueous iodide has an intense yellow to brown colour. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Starch solution is used for end point detection in iodometric titration. One drop of 0,05 m iodine solution. As has been mentioned above,. Iodometric Titration End Point.
From www.slideserve.com
PPT Objectives PowerPoint Presentation, free download ID2145720 Iodometric Titration End Point Starch solution is used for end point detection in iodometric titration. The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. Add 50 ml of deionized. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. One drop of 0,05 m iodine solution. To prepare. Iodometric Titration End Point.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Iodometric Titration End Point The endpoint of the titration is located with the starch indicator solution that was also prepared earlier. Starch solution is used for end point detection in iodometric titration. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. To prepare starch indicator solution, add 1 gram of starch (either. One drop of. Iodometric Titration End Point.