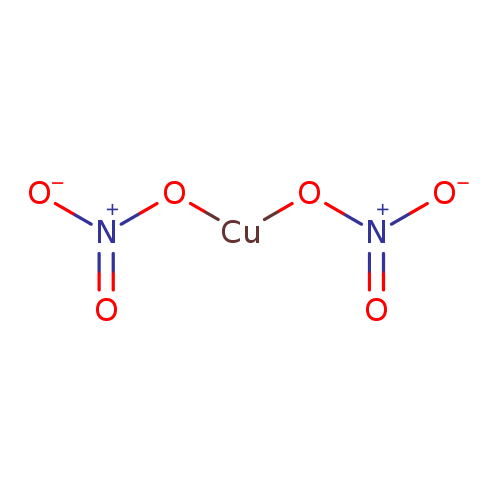
Copper Ii Nitrate Acid Or Base . Indicators are used to determine whether a solution is acidic or alkaline. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g) + 2h 2 o(l) the reaction. Copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. Acids react with metals, bases and carbonates to produce salts. If it is a simple molecule we use greek prefixes to identify the number.
from www.t3db.ca
Copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g) + 2h 2 o(l) the reaction. Acids react with metals, bases and carbonates to produce salts. Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. Indicators are used to determine whether a solution is acidic or alkaline. If it is a simple molecule we use greek prefixes to identify the number. If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules.
T3DB Copper(II) nitrate
Copper Ii Nitrate Acid Or Base If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. If it is a simple molecule we use greek prefixes to identify the number. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Indicators are used to determine whether a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g) + 2h 2 o(l) the reaction. If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. Copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water.
From www.sciencemadness.org
Copper(II) nitrate Sciencemadness Wiki Copper Ii Nitrate Acid Or Base If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. Acids react with metals, bases and carbonates to produce salts. Indicators are used to determine whether a solution is acidic or alkaline. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or. Copper Ii Nitrate Acid Or Base.
From www.carolina.com
Copper (II) Nitrate, Trihydrate, Reagent Grade, 100 g Copper Ii Nitrate Acid Or Base If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2. Copper Ii Nitrate Acid Or Base.
From www.youtube.com
Copper and Nitric acid reaction YouTube Copper Ii Nitrate Acid Or Base Indicators are used to determine whether a solution is acidic or alkaline. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g) + 2h 2 o(l) the reaction. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Copper reacts with nitric acid, forming. Copper Ii Nitrate Acid Or Base.
From www.indiamart.com
Copper Ii Nitrate Trihydrate at best price in Mumbai by Cosmic Copper Ii Nitrate Acid Or Base Copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g) + 2h 2 o(l) the reaction. Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. Indicators are used to determine. Copper Ii Nitrate Acid Or Base.
From www.youtube.com
Copper metal reacts with nitric acid to form a solution of copper(II Copper Ii Nitrate Acid Or Base Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. If it is a simple molecule we use greek prefixes to identify the number. If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid.. Copper Ii Nitrate Acid Or Base.
From www.selectschoolsupplies.co.uk
Copper II Nitrate Trihydrate 99.5 ACS 500g Copper Ii Nitrate Acid Or Base If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. Acids react with metals, bases and carbonates to produce salts. If it is a. Copper Ii Nitrate Acid Or Base.
From hoachathoangha.com.vn
Copper (II) nitrate trihydrate Copper Ii Nitrate Acid Or Base Copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Acids react with metals, bases and carbonates to produce salts. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g). Copper Ii Nitrate Acid Or Base.
From www.numerade.com
SOLVED Part 1 [Copper metal to Copper (II) nitrate] 2pts. Balanced Copper Ii Nitrate Acid Or Base If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. If it is a simple molecule we use greek prefixes to identify the number. Acids react with metals, bases and carbonates to produce salts. Indicators are used to determine whether a solution is acidic. Copper Ii Nitrate Acid Or Base.
From www.numerade.com
SOLVED Write a balanced chemical equation for the following reaction Copper Ii Nitrate Acid Or Base Acids react with metals, bases and carbonates to produce salts. Copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can. Copper Ii Nitrate Acid Or Base.
From www.pinterest.com
Copper (II) Nitrate Trihydrate, 500g LabGrade The Curated Chemical Copper Ii Nitrate Acid Or Base Indicators are used to determine whether a solution is acidic or alkaline. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. Copper reacts with nitric acid, forming aqueous. Copper Ii Nitrate Acid Or Base.
From khushaty.blogspot.com
Copper Ii Nitrate Formula T3DB Barium nitrate What are the correct Copper Ii Nitrate Acid Or Base If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. Acids react with metals, bases and carbonates to produce salts. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Copper (ii) nitrate. Copper Ii Nitrate Acid Or Base.
From www.numerade.com
SOLVED When copper(II) oxide reacts with dilute nitric acid, copper(II Copper Ii Nitrate Acid Or Base To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. If it is a simple molecule we use greek prefixes to identify the number. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g) + 2h 2 o(l) the reaction. Copper (ii) nitrate can. Copper Ii Nitrate Acid Or Base.
From sciencenotes.org
Copper and Nitric Acid Chemistry Demonstration Copper Ii Nitrate Acid Or Base To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. If it is a simple molecule we use greek prefixes to identify the number. Indicators are used to determine whether a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts. Copper reacts with. Copper Ii Nitrate Acid Or Base.
From www.restauro-online.com
Copper(II) nitrate trihydrate pure (Cu(NO3)2 · 3H2O) buy online Copper Ii Nitrate Acid Or Base Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g) + 2h 2 o(l) the reaction. If it is a simple molecule we use greek prefixes to identify the number. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. If it is covalent,. Copper Ii Nitrate Acid Or Base.
From lab.honeywell.com
Copper(II) nitrate trihydrate 61194 Honeywell Research Chemicals Copper Ii Nitrate Acid Or Base If it is a simple molecule we use greek prefixes to identify the number. If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple. Copper Ii Nitrate Acid Or Base.
From www.flinnsci.com
Flinn Chemicals, Copper(II) Nitrate Solution Copper Ii Nitrate Acid Or Base If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and. Copper Ii Nitrate Acid Or Base.
From www.biosynth.com
FC106444 10031433 Copper(II) nitrate trihydrate Copper Ii Nitrate Acid Or Base Indicators are used to determine whether a solution is acidic or alkaline. Copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Acids react with metals, bases and carbonates to produce salts. If it is. Copper Ii Nitrate Acid Or Base.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Ii Nitrate Acid Or Base Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. Copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. Acids react with metals, bases and carbonates to produce salts. If it is a simple molecule we use greek prefixes to identify the number. Indicators are. Copper Ii Nitrate Acid Or Base.
From testbook.com
Copper (II) Nitrate Formula Learn Structure, Properties, Uses Copper Ii Nitrate Acid Or Base Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g) + 2h 2 o(l) the reaction. If it is a simple molecule we use greek prefixes to identify the number. Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. Copper reacts with nitric acid,. Copper Ii Nitrate Acid Or Base.
From www.t3db.ca
T3DB Copper(II) nitrate Copper Ii Nitrate Acid Or Base If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. If it is a simple molecule we use greek prefixes to identify the number. Indicators are used to determine whether a solution is acidic or alkaline. Copper (ii) nitrate can be prepared by reacting. Copper Ii Nitrate Acid Or Base.
From www.alamy.com
3D image of Copper II nitrate skeletal formula molecular chemical Copper Ii Nitrate Acid Or Base Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. If it is a simple molecule we use greek prefixes to identify the number. Cu(s) + 4hno 3 (aq). Copper Ii Nitrate Acid Or Base.
From www.carolina.com
Copper (II) Nitrate, Trihydrate, Reagent Grade, 25 g Copper Ii Nitrate Acid Or Base Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. Indicators are used to determine whether a solution is acidic or alkaline. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Cu(s) + 4hno 3 (aq) → cu(no. Copper Ii Nitrate Acid Or Base.
From www.sciencephoto.com
Copper(II) nitrate Stock Image C043/2653 Science Photo Library Copper Ii Nitrate Acid Or Base If it is a simple molecule we use greek prefixes to identify the number. If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. Acids react with metals, bases and carbonates to produce salts. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2. Copper Ii Nitrate Acid Or Base.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Ii Nitrate Acid Or Base Indicators are used to determine whether a solution is acidic or alkaline. Copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. Copper (ii) nitrate can be prepared by reacting. Copper Ii Nitrate Acid Or Base.
From www.youtube.com
How to Write the Formula for Copper (II) nitrate YouTube Copper Ii Nitrate Acid Or Base Acids react with metals, bases and carbonates to produce salts. If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. Cu(s) + 4hno 3. Copper Ii Nitrate Acid Or Base.
From www.sciencephoto.com
Copper(II) nitrate Stock Image C043/2658 Science Photo Library Copper Ii Nitrate Acid Or Base To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no. Copper Ii Nitrate Acid Or Base.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Copper Ii Nitrate Acid Or Base If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. Indicators are used to determine whether a solution is acidic or alkaline. Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. Copper reacts. Copper Ii Nitrate Acid Or Base.
From www.carolina.com
Copper (II) Nitrate Solution, 0.1 M, Laboratory Grade, 500 mL Copper Ii Nitrate Acid Or Base If it is a simple molecule we use greek prefixes to identify the number. Acids react with metals, bases and carbonates to produce salts. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g) + 2h 2 o(l) the reaction. Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the. Copper Ii Nitrate Acid Or Base.
From www.flinnsci.com
Flinn Chemicals, Copper(II) Nitrate Copper Ii Nitrate Acid Or Base To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g) + 2h 2 o(l) the reaction. Acids react with metals, bases and carbonates to produce salts. If it is a simple molecule we use. Copper Ii Nitrate Acid Or Base.
From sciencenotes.org
Copper and Nitric Acid Chemistry Demonstration Copper Ii Nitrate Acid Or Base If it is a simple molecule we use greek prefixes to identify the number. Copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. Acids react with metals, bases and carbonates to produce salts. Indicators are used to determine whether a solution is acidic or alkaline. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic. Copper Ii Nitrate Acid Or Base.
From www.southernbiological.com
Copper (II) Nitrate, 0.1M, Laboratory Grade Southern Biological Copper Ii Nitrate Acid Or Base To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Copper (ii) nitrate can be prepared by reacting copper with concentrated nitric acid, forming the compound and releasing nitrogen dioxide. If it is a simple molecule we use greek prefixes to identify the number. If it is covalent, which. Copper Ii Nitrate Acid Or Base.
From www.thermofisher.com
Copper(II) nitrate trihydrate, 99, pure, Thermo Scientific™ Copper Ii Nitrate Acid Or Base If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Acids react with metals, bases and carbonates to produce salts. Copper (ii) nitrate. Copper Ii Nitrate Acid Or Base.
From excichem.com
10031433CopperIINitrateTrihydrateAR500 Excichem Research Copper Ii Nitrate Acid Or Base If it is a simple molecule we use greek prefixes to identify the number. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g) + 2h 2 o(l) the reaction. Copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline),. Copper Ii Nitrate Acid Or Base.
From www.numerade.com
SOLVED The reaction between solid copper and nitric acid to form Copper Ii Nitrate Acid Or Base If it is a simple molecule we use greek prefixes to identify the number. To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Indicators are used to determine whether a solution is acidic or alkaline. If it is covalent, which is typically between 2 or more nonmetals, we. Copper Ii Nitrate Acid Or Base.
From www.youtube.com
Number of Ions in Cu(NO3)2 Copper (II) nitrate YouTube Copper Ii Nitrate Acid Or Base To tell if cu(no3)2 (copper nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules. Copper reacts with nitric acid, forming aqueous copper nitrate, nitrogen dioxide gas, and water. Cu(s) + 4hno 3 (aq) → cu(no 3 ) 2 (aq) + 2no 2 (g) + 2h 2 o(l) the reaction. Indicators are used to. Copper Ii Nitrate Acid Or Base.