Standard Reduction Potential Half Reactions . assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Use standard reduction potentials to determine the.
from www.numerade.com
assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Use standard reduction potentials to determine the. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices.
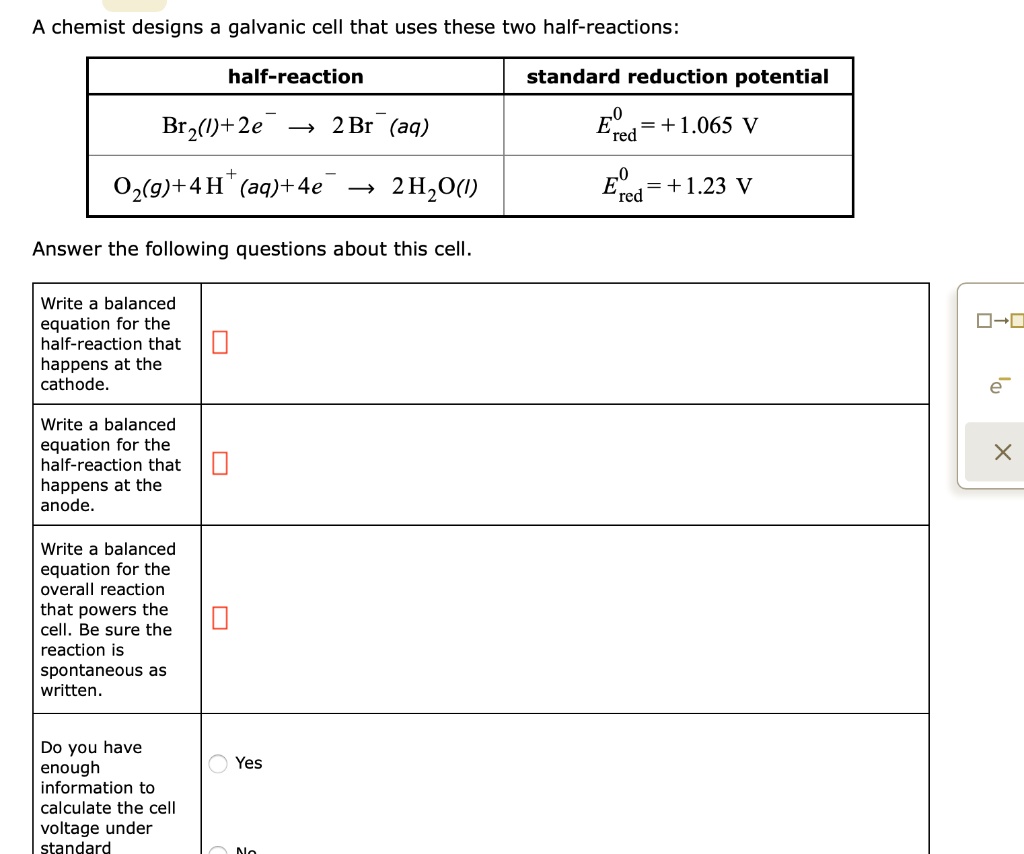
SOLVEDA chemist designs a galvanic cell that uses these two half
Standard Reduction Potential Half Reactions assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Use standard reduction potentials to determine the. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard.
From www.chegg.com
Solved Standard Redox Potentials of Selected HalfReactions Standard Reduction Potential Half Reactions Use standard reduction potentials to determine the. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the table is ordered such that the stronger (more reactive) reductants are at the top and. Standard Reduction Potential Half Reactions.
From www.coursehero.com
[Solved] Consider the following two halfreactions and their standard Standard Reduction Potential Half Reactions the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Use standard reduction potentials to determine the. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Half Reactions.
From www.numerade.com
SOLVED A chemist designs a galvanic cell that uses these two half Standard Reduction Potential Half Reactions the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Use standard reduction potentials to determine the. assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Half Reactions.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Reduction Potential Half Reactions assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. Use standard reduction potentials to determine the. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or. Standard Reduction Potential Half Reactions.
From www.chegg.com
Solved Standard Reduction Potentials at 25°C (298 K) for Standard Reduction Potential Half Reactions assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices.. Standard Reduction Potential Half Reactions.
From www.slideserve.com
PPT The oxidation states of vanadium PowerPoint Presentation ID467926 Standard Reduction Potential Half Reactions the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Use standard reduction potentials to determine the. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. use standard reduction potentials to determine the better oxidizing or. Standard Reduction Potential Half Reactions.
From pressbooks.uiowa.edu
Topic 11 Appendix A Standard Reduction Potentials at 25ºC CHEM 1120 Standard Reduction Potential Half Reactions assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. Use standard reduction potentials to determine the. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. the table is ordered such that the stronger (more reactive) reductants are at the top and. Standard Reduction Potential Half Reactions.
From www.pveducation.org
Standard Potential PVEducation Standard Reduction Potential Half Reactions the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. Use standard reduction potentials to determine the. use standard reduction potentials to determine the better oxidizing or. Standard Reduction Potential Half Reactions.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281515 Standard Reduction Potential Half Reactions use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Use standard reduction potentials to determine the. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Half Reactions.
From www.chegg.com
Solved Using the table of standard reduction potentials at Standard Reduction Potential Half Reactions the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Use standard reduction potentials to determine the. assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Half Reactions.
From www.numerade.com
SOLVEDA chemist designs a galvanic cell that uses these two half Standard Reduction Potential Half Reactions assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices.. Standard Reduction Potential Half Reactions.
From www.youtube.com
Standard Reduction Potentials of Half Reactions Electrochemistry Standard Reduction Potential Half Reactions Use standard reduction potentials to determine the. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the table is ordered such that the stronger (more reactive) reductants are at the top and. Standard Reduction Potential Half Reactions.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Half Reactions assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Use standard reduction potentials to determine the. the table is ordered such that the stronger (more reactive) reductants are at the top and. Standard Reduction Potential Half Reactions.
From byjus.com
The standard reduction potential of the reaction at 25^° C ,Н2О + e Standard Reduction Potential Half Reactions Use standard reduction potentials to determine the. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the table is ordered such that the stronger (more reactive) reductants are at the top and. Standard Reduction Potential Half Reactions.
From slideplayer.com
Electrochemistry Part III Reduction Potentials ppt download Standard Reduction Potential Half Reactions the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard.. Standard Reduction Potential Half Reactions.
From ch302.cm.utexas.edu
stdpotsshortlist.png Standard Reduction Potential Half Reactions Use standard reduction potentials to determine the. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Half Reactions.
From www.numerade.com
SOLVED TABLE 137b Standard Reduction Potentials of Some Biologically Standard Reduction Potential Half Reactions Use standard reduction potentials to determine the. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Half Reactions.
From www.numerade.com
SOLVED Table 2. Table of Selected Standard Reduction Potentials at 25Â Standard Reduction Potential Half Reactions Use standard reduction potentials to determine the. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Half Reactions.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Half Reactions the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices.. Standard Reduction Potential Half Reactions.
From oneclass.com
OneClass Given the following halfreactions and their respective Standard Reduction Potential Half Reactions assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom.. Standard Reduction Potential Half Reactions.
From ch302.cm.utexas.edu
Electrochemistry_Reduction_Potentials Standard Reduction Potential Half Reactions Use standard reduction potentials to determine the. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Half Reactions.
From askfilo.com
Standard reduction potential of half reactions are given below Au3+(aq)+3.. Standard Reduction Potential Half Reactions the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices.. Standard Reduction Potential Half Reactions.
From www.chegg.com
Solved A chemist designs a galvanic cell that uses these two Standard Reduction Potential Half Reactions use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Use standard reduction potentials to determine the. assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Half Reactions.
From www.doubtnut.com
Standard reduction potential of the half reactions are given below Standard Reduction Potential Half Reactions Use standard reduction potentials to determine the. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the table is ordered such that the stronger (more reactive) reductants are at the top and. Standard Reduction Potential Half Reactions.
From www.chegg.com
Solved Table 1. Standard Reduction Potentials HalfReactions Standard Reduction Potential Half Reactions Use standard reduction potentials to determine the. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. the table is ordered such that the stronger (more reactive) reductants are at the top and. Standard Reduction Potential Half Reactions.
From www.pinterest.com
Standard Reduction Potential (E) when given two half reactions and Standard Reduction Potential Half Reactions the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard.. Standard Reduction Potential Half Reactions.
From www.numerade.com
SOLVED The standard reduction potentials of the following half Standard Reduction Potential Half Reactions assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices.. Standard Reduction Potential Half Reactions.
From www.flinnsci.com
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Half Reactions Use standard reduction potentials to determine the. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the table is ordered such that the stronger (more reactive) reductants are at the top and. Standard Reduction Potential Half Reactions.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Atoms First Standard Reduction Potential Half Reactions the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Use standard reduction potentials to determine the. assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Half Reactions.
From www.numerade.com
SOLVED Selective Reduction The standard reduction potential for the Standard Reduction Potential Half Reactions the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard.. Standard Reduction Potential Half Reactions.
From www.chegg.com
Solved 5. Use The Standard Redox Table 11.1 (on Page 1) T... Standard Reduction Potential Half Reactions use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Use standard reduction potentials to determine the. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Half Reactions.
From www.bartleby.com
Answered Selected Standard Reduction Potentials… bartleby Standard Reduction Potential Half Reactions assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Use standard reduction potentials to determine the. the table is ordered such that the stronger (more reactive) reductants are at the top and. Standard Reduction Potential Half Reactions.
From courses.lumenlearning.com
Standard Reduction Potentials General Chemistry Standard Reduction Potential Half Reactions use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Use standard reduction potentials to determine the. assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Half Reactions.
From www.slideserve.com
PPT Figure 161 Map of the major metabolic pathways in a typical cell Standard Reduction Potential Half Reactions Use standard reduction potentials to determine the. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Half Reactions.
From chemistrynotes.com
Cell Potential Calculations and Line Notation Standard Reduction Potential Half Reactions assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. Use standard reduction potentials to determine the. the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. use standard reduction potentials to determine the better oxidizing or. Standard Reduction Potential Half Reactions.