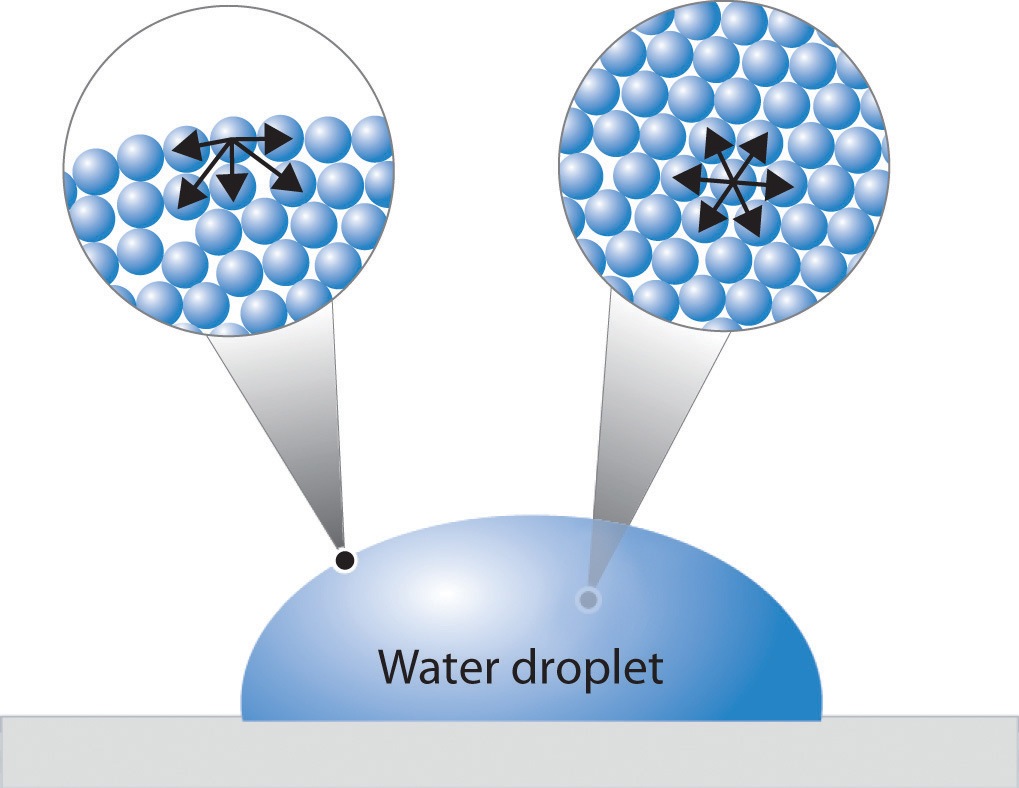
What Does It Mean To Have A High Surface Tension . surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a manifestation. How does polarity affect it. next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Check out a few diagrams. what is surface tension. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. The cohesive forces between liquid molecules. Learn the surface tension of water.
from chem.libretexts.org
liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Surface tension is a manifestation. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. what is surface tension. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The cohesive forces between liquid molecules. Check out a few diagrams. next to mercury, water has the highest surface tension of all commonly occurring liquids. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. How does polarity affect it.
Surface Tension Chemistry LibreTexts
What Does It Mean To Have A High Surface Tension what is surface tension. Learn the surface tension of water. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. what is surface tension. Surface tension is a manifestation. next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Check out a few diagrams. The cohesive forces between liquid molecules. How does polarity affect it.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it What Does It Mean To Have A High Surface Tension surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. How does polarity affect it. Check out a few diagrams. Learn the surface tension of water. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. next to mercury, water. What Does It Mean To Have A High Surface Tension.
From www.vrogue.co
Surface Tension Definition Examples Causes And Measur vrogue.co What Does It Mean To Have A High Surface Tension Surface tension is a manifestation. what is surface tension. next to mercury, water has the highest surface tension of all commonly occurring liquids. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. surface tension is the energy, or work, required to increase the surface area of a. What Does It Mean To Have A High Surface Tension.
From www.youtube.com
Surfactant and Surface Tension in Respiration Breathing Mechanics What Does It Mean To Have A High Surface Tension what is surface tension. The cohesive forces between liquid molecules. How does polarity affect it. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. next to mercury, water has the highest surface tension of all commonly occurring liquids. Check out a few diagrams. surface tension is the. What Does It Mean To Have A High Surface Tension.
From hxeafvitb.blob.core.windows.net
Why Water Have High Surface Tension at Nancy Aguirre blog What Does It Mean To Have A High Surface Tension liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules. Learn the surface. What Does It Mean To Have A High Surface Tension.
From learningmediawashermen.z14.web.core.windows.net
How To Explain Surface Tension To Kids What Does It Mean To Have A High Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. what is surface tension. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. How does polarity affect it. The cohesive forces between liquid molecules. Learn the surface tension. What Does It Mean To Have A High Surface Tension.
From www.vrogue.co
What Is Surface Tension Molecular Explanation vrogue.co What Does It Mean To Have A High Surface Tension water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. next to mercury, water has the highest surface tension of all commonly occurring liquids. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. surface tension, property of a liquid surface displayed by its. What Does It Mean To Have A High Surface Tension.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID1714027 What Does It Mean To Have A High Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. The cohesive forces between liquid molecules. what is surface tension. How does polarity affect it. surface tension, property. What Does It Mean To Have A High Surface Tension.
From worksheethaunches.z14.web.core.windows.net
What Has Surface Tension What Does It Mean To Have A High Surface Tension Surface tension is a manifestation. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. what is surface tension. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Learn the surface tension of water. How does polarity affect it. Check out a few diagrams.. What Does It Mean To Have A High Surface Tension.
From hxeafvitb.blob.core.windows.net
Why Water Have High Surface Tension at Nancy Aguirre blog What Does It Mean To Have A High Surface Tension liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. How does polarity affect it. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. what is surface tension. Learn the surface tension of water. surface tension, property of a liquid surface displayed by. What Does It Mean To Have A High Surface Tension.
From exydwggxr.blob.core.windows.net
What Is A High Or Low Surface Tension at Michael Moore blog What Does It Mean To Have A High Surface Tension liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Surface tension is a manifestation. what is surface tension. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Learn the surface tension of water. surface tension is the energy, or work, required to. What Does It Mean To Have A High Surface Tension.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL What Does It Mean To Have A High Surface Tension what is surface tension. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. The cohesive forces between liquid molecules. Learn the surface tension of water. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Check out a. What Does It Mean To Have A High Surface Tension.
From www.researchgate.net
a wetting state of surface with low and highsurfacetension fluids b What Does It Mean To Have A High Surface Tension water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. How does polarity affect it. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is a manifestation. surface tension is the energy, or work, required to increase. What Does It Mean To Have A High Surface Tension.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Does It Mean To Have A High Surface Tension next to mercury, water has the highest surface tension of all commonly occurring liquids. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Surface tension is a manifestation. How does polarity affect it. surface tension, property of a liquid surface displayed by its acting as if it were. What Does It Mean To Have A High Surface Tension.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Does It Mean To Have A High Surface Tension The cohesive forces between liquid molecules. How does polarity affect it. Learn the surface tension of water. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. next to mercury, water has the highest surface tension of all commonly occurring liquids. what is surface tension. Surface tension is a. What Does It Mean To Have A High Surface Tension.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts What Does It Mean To Have A High Surface Tension what is surface tension. The cohesive forces between liquid molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. water has a high surface tension because. What Does It Mean To Have A High Surface Tension.
From www.youtube.com
How does Surface Tension work? YouTube What Does It Mean To Have A High Surface Tension Check out a few diagrams. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a manifestation. Learn the surface tension of water. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. How does polarity affect it. surface tension,. What Does It Mean To Have A High Surface Tension.
From guidemanualspyglasses.z14.web.core.windows.net
How To Explain Surface Tension What Does It Mean To Have A High Surface Tension How does polarity affect it. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Check out a few diagrams. Learn the surface tension of water. next to mercury, water has the highest surface tension of all commonly occurring liquids. water has a high surface tension because hydrogen. What Does It Mean To Have A High Surface Tension.
From exydwggxr.blob.core.windows.net
What Is A High Or Low Surface Tension at Michael Moore blog What Does It Mean To Have A High Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. The cohesive forces between liquid. What Does It Mean To Have A High Surface Tension.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Does It Mean To Have A High Surface Tension Learn the surface tension of water. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance.. What Does It Mean To Have A High Surface Tension.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Does It Mean To Have A High Surface Tension The cohesive forces between liquid molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Learn the surface tension of water. next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a manifestation. what is surface tension. liquids. What Does It Mean To Have A High Surface Tension.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Does It Mean To Have A High Surface Tension Surface tension is a manifestation. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. next to mercury, water has the highest surface tension of all commonly occurring. What Does It Mean To Have A High Surface Tension.
From hxedgviga.blob.core.windows.net
What Causes High Surface Tension Of Water at Todd James blog What Does It Mean To Have A High Surface Tension liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Surface tension is a manifestation. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. How does polarity affect it. what is surface tension. next to mercury, water has the highest surface. What Does It Mean To Have A High Surface Tension.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Does It Mean To Have A High Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. surface tension, property of. What Does It Mean To Have A High Surface Tension.
From cesioues.blob.core.windows.net
High Surface Tension Facts at Margaret Halpern blog What Does It Mean To Have A High Surface Tension How does polarity affect it. what is surface tension. Check out a few diagrams. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Learn the surface tension of water. next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a manifestation. surface tension is. What Does It Mean To Have A High Surface Tension.
From williamgbutts.blob.core.windows.net
What Does High Surface Tension Cause at williamgbutts blog What Does It Mean To Have A High Surface Tension surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a manifestation. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. water has a high. What Does It Mean To Have A High Surface Tension.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download What Does It Mean To Have A High Surface Tension liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. next to mercury, water has the highest surface tension of all commonly occurring liquids. Learn the surface tension of water. The cohesive forces between liquid molecules. Check out a few diagrams. water has a high surface tension because hydrogen bonds among water molecules. What Does It Mean To Have A High Surface Tension.
From eduinput.com
Surface TensionDefinition, Examples, Causes, And Measurement What Does It Mean To Have A High Surface Tension water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a manifestation. How does polarity affect it. surface tension is the energy, or work, required to increase the surface area of a. What Does It Mean To Have A High Surface Tension.
From slideplayer.com
Chapter 2 The Chemistry of Life ppt download What Does It Mean To Have A High Surface Tension water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Learn the surface tension of water. How does polarity affect it. Surface tension is a manifestation. Check out a few diagrams. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. next to mercury, water. What Does It Mean To Have A High Surface Tension.
From vectormine.com
Surface tension explanation vector illustration diagram VectorMine What Does It Mean To Have A High Surface Tension next to mercury, water has the highest surface tension of all commonly occurring liquids. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. How does polarity affect it. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. The cohesive forces between liquid molecules.. What Does It Mean To Have A High Surface Tension.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Does It Mean To Have A High Surface Tension Learn the surface tension of water. Check out a few diagrams. Surface tension is a manifestation. How does polarity affect it. next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The cohesive forces between. What Does It Mean To Have A High Surface Tension.
From byjus.com
Explain the surface tension phenomenon with examples. What Does It Mean To Have A High Surface Tension The cohesive forces between liquid molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Check out a few diagrams. water has a high surface tension because. What Does It Mean To Have A High Surface Tension.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3227401 What Does It Mean To Have A High Surface Tension How does polarity affect it. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. The cohesive forces between liquid molecules. surface tension, property of a liquid surface displayed. What Does It Mean To Have A High Surface Tension.
From www.vrogue.co
What Are The Importances Of Surface Tension And Visco vrogue.co What Does It Mean To Have A High Surface Tension How does polarity affect it. next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a manifestation. water has a high surface tension because hydrogen bonds among water molecules resist. What Does It Mean To Have A High Surface Tension.
From hxetjoglu.blob.core.windows.net
What Is Meant By A High Surface Tension at Shanelle Choy blog What Does It Mean To Have A High Surface Tension Check out a few diagrams. water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. what is surface tension. Surface tension is a manifestation. The cohesive forces between liquid molecules. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. How does polarity affect it.. What Does It Mean To Have A High Surface Tension.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Does It Mean To Have A High Surface Tension water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Learn the surface tension of water. what is surface tension. surface tension, property of a liquid surface displayed by its acting as if it. What Does It Mean To Have A High Surface Tension.