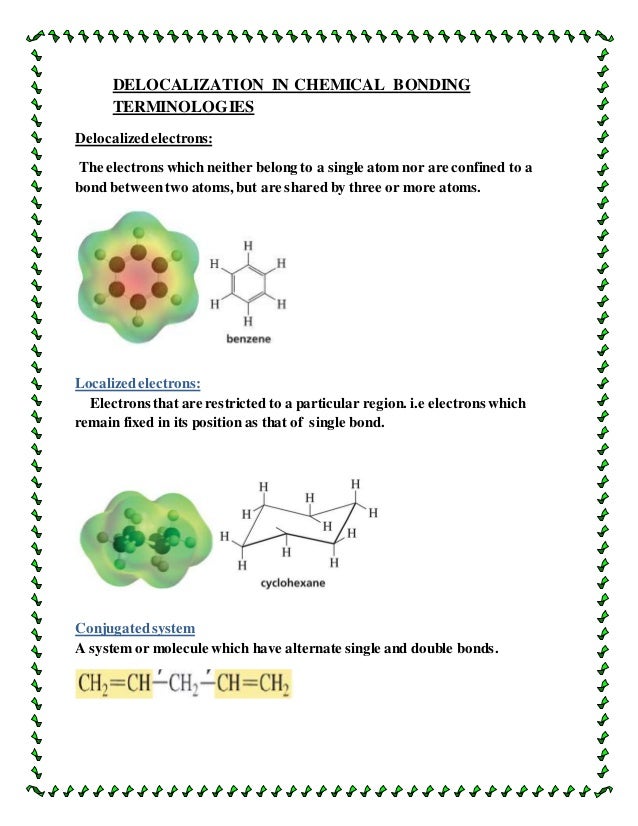
What Is The Meaning Of Delocalised . That is, the orbitals spread over the entire molecule. delocalized electrons are not associated with any particular atom. For example, bonding electrons may be distributed among several atoms. a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles of electron movement used in writing resonance structures in lewis notation, known as the curved arrow formalism. delocalization happens when electric charge is spread over more than one atom. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. Localized electrons are found between atoms and are confined.
from www.slideshare.net
delocalization happens when electric charge is spread over more than one atom. That is, the orbitals spread over the entire molecule. delocalized electrons are not associated with any particular atom. Localized electrons are found between atoms and are confined. a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles of electron movement used in writing resonance structures in lewis notation, known as the curved arrow formalism. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. For example, bonding electrons may be distributed among several atoms.
Delocalization in chemical bonding
What Is The Meaning Of Delocalised delocalization happens when electric charge is spread over more than one atom. delocalization happens when electric charge is spread over more than one atom. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles of electron movement used in writing resonance structures in lewis notation, known as the curved arrow formalism. delocalized electrons are not associated with any particular atom. Localized electrons are found between atoms and are confined. a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. That is, the orbitals spread over the entire molecule. For example, bonding electrons may be distributed among several atoms.
From www.chemistrysteps.com
Localized and Delocalized Lone Pairs and Bonds Chemistry Steps What Is The Meaning Of Delocalised That is, the orbitals spread over the entire molecule. Localized electrons are found between atoms and are confined. a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship. What Is The Meaning Of Delocalised.
From www.thoughtco.com
A Delocalized Electron Defined in Chemistry What Is The Meaning Of Delocalised delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. to. What Is The Meaning Of Delocalised.
From www.thoughtco.com
Delocalized Electron Definition in Chemistry What Is The Meaning Of Delocalised Localized electrons are found between atoms and are confined. delocalized electrons are not associated with any particular atom. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. That is, the orbitals spread over the. What Is The Meaning Of Delocalised.
From www.youtube.com
04.04 Stability Factors Resonance Delocalization YouTube What Is The Meaning Of Delocalised delocalization happens when electric charge is spread over more than one atom. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. That is, the orbitals spread over the entire molecule. For example, bonding electrons. What Is The Meaning Of Delocalised.
From www.slideserve.com
PPT Delocalized Electrons Resonance PowerPoint Presentation ID6519040 What Is The Meaning Of Delocalised For example, bonding electrons may be distributed among several atoms. That is, the orbitals spread over the entire molecule. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. Localized electrons are found between atoms and. What Is The Meaning Of Delocalised.
From www.slideserve.com
PPT Chemistry 100 Chapter 9 PowerPoint Presentation, free download What Is The Meaning Of Delocalised delocalization happens when electric charge is spread over more than one atom. delocalized electrons are not associated with any particular atom. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. a delocalized. What Is The Meaning Of Delocalised.
From www.youtube.com
14.1 Molecules and ions with delocalised pi electrons (HL) YouTube What Is The Meaning Of Delocalised That is, the orbitals spread over the entire molecule. delocalized electrons are not associated with any particular atom. Localized electrons are found between atoms and are confined. a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. delocalization happens when electric charge is spread. What Is The Meaning Of Delocalised.
From www.slideserve.com
PPT The Delocalized Approach to Bonding Molecular Orbital Theory What Is The Meaning Of Delocalised For example, bonding electrons may be distributed among several atoms. delocalization happens when electric charge is spread over more than one atom. That is, the orbitals spread over the entire molecule. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles. What Is The Meaning Of Delocalised.
From www.thesciencehive.co.uk
Aromatic compounds — the science hive What Is The Meaning Of Delocalised Localized electrons are found between atoms and are confined. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. That is, the orbitals spread over the entire molecule. delocalized electrons are not associated with any. What Is The Meaning Of Delocalised.
From www.slideserve.com
PPT Chapter 9 PowerPoint Presentation ID4493524 What Is The Meaning Of Delocalised to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles of electron movement used in writing resonance structures in lewis notation, known as the curved arrow formalism. a delocalized electron is an electron in an atom, ion, or molecule not associated. What Is The Meaning Of Delocalised.
From www.youtube.com
Localised Bond and Delocalised Bondwhat is the Localised Bond and What Is The Meaning Of Delocalised delocalization happens when electric charge is spread over more than one atom. Localized electrons are found between atoms and are confined. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. to introduce the. What Is The Meaning Of Delocalised.
From www.youtube.com
Delocalized Meaning YouTube What Is The Meaning Of Delocalised That is, the orbitals spread over the entire molecule. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles of electron movement used in writing resonance structures in lewis notation, known as the curved arrow formalism. delocalization happens when electric charge. What Is The Meaning Of Delocalised.
From www.youtube.com
14.1 Delocalized pi electrons (HL) YouTube What Is The Meaning Of Delocalised For example, bonding electrons may be distributed among several atoms. delocalized electrons are not associated with any particular atom. That is, the orbitals spread over the entire molecule. Localized electrons are found between atoms and are confined. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts. What Is The Meaning Of Delocalised.
From www.youtube.com
Delocalised Structure of Benzene YouTube What Is The Meaning Of Delocalised delocalized electrons are not associated with any particular atom. Localized electrons are found between atoms and are confined. That is, the orbitals spread over the entire molecule. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently. What Is The Meaning Of Delocalised.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID3391055 What Is The Meaning Of Delocalised That is, the orbitals spread over the entire molecule. delocalization happens when electric charge is spread over more than one atom. delocalized electrons are not associated with any particular atom. a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. to introduce the. What Is The Meaning Of Delocalised.
From www.youtube.com
Delocalized electron YouTube What Is The Meaning Of Delocalised Localized electrons are found between atoms and are confined. For example, bonding electrons may be distributed among several atoms. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles of electron movement used in writing resonance structures in lewis notation, known as. What Is The Meaning Of Delocalised.
From www.slideserve.com
PPT 14.1 Shapes of molecules and ions (HL) PowerPoint Presentation What Is The Meaning Of Delocalised delocalization happens when electric charge is spread over more than one atom. For example, bonding electrons may be distributed among several atoms. That is, the orbitals spread over the entire molecule. Localized electrons are found between atoms and are confined. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective. What Is The Meaning Of Delocalised.
From www.youtube.com
Identifying delocalized electrons example YouTube What Is The Meaning Of Delocalised delocalization happens when electric charge is spread over more than one atom. a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. Localized electrons are found between atoms and are confined. to introduce the concept of electron delocalization from the perspective of molecular orbitals,. What Is The Meaning Of Delocalised.
From slideplayer.com
Organic molecular structure ppt download What Is The Meaning Of Delocalised a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. delocalized electrons are not associated with any particular atom. delocalization happens when electric charge is spread over more than one atom. For example, bonding electrons may be distributed among several atoms. delocalisation of. What Is The Meaning Of Delocalised.
From www.differencebetween.com
Difference Between Localized and Delocalized Electrons Compare the What Is The Meaning Of Delocalised For example, bonding electrons may be distributed among several atoms. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. delocalized electrons are not associated with any particular atom. to introduce the concept of. What Is The Meaning Of Delocalised.
From www.youtube.com
delocalized vs localized lone pairs YouTube What Is The Meaning Of Delocalised Localized electrons are found between atoms and are confined. delocalized electrons are not associated with any particular atom. a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. For example, bonding electrons may be distributed among several atoms. That is, the orbitals spread over the. What Is The Meaning Of Delocalised.
From www.youtube.com
Organic Chemistry Notes 3.1 Localized vs. Delocalized Electrons YouTube What Is The Meaning Of Delocalised delocalized electrons are not associated with any particular atom. For example, bonding electrons may be distributed among several atoms. That is, the orbitals spread over the entire molecule. Localized electrons are found between atoms and are confined. a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single. What Is The Meaning Of Delocalised.
From www.chemistrystudent.com
Metallic Bonding (ALevel) ChemistryStudent What Is The Meaning Of Delocalised For example, bonding electrons may be distributed among several atoms. Localized electrons are found between atoms and are confined. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. That is, the orbitals spread over the. What Is The Meaning Of Delocalised.
From chem.libretexts.org
Delocalization of Electrons Chemistry LibreTexts What Is The Meaning Of Delocalised That is, the orbitals spread over the entire molecule. delocalized electrons are not associated with any particular atom. Localized electrons are found between atoms and are confined. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles of electron movement used. What Is The Meaning Of Delocalised.
From www.nagwa.com
Question Video Defining Delocalized Electrons Nagwa What Is The Meaning Of Delocalised That is, the orbitals spread over the entire molecule. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles of electron movement used in writing resonance structures in lewis notation, known as the curved arrow formalism. Localized electrons are found between atoms. What Is The Meaning Of Delocalised.
From studylib.net
Delocalization of Electrons What Is The Meaning Of Delocalised For example, bonding electrons may be distributed among several atoms. That is, the orbitals spread over the entire molecule. delocalized electrons are not associated with any particular atom. Localized electrons are found between atoms and are confined. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and. What Is The Meaning Of Delocalised.
From www.slideshare.net
Delocalization in chemical bonding What Is The Meaning Of Delocalised Localized electrons are found between atoms and are confined. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. a delocalized electron is an electron in an atom, ion, or molecule not associated with any. What Is The Meaning Of Delocalised.
From www.sliderbase.com
Sigma and Pi Bonds What Is The Meaning Of Delocalised That is, the orbitals spread over the entire molecule. delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. For example, bonding electrons may be distributed among several atoms. to introduce the concept of electron. What Is The Meaning Of Delocalised.
From www.chemistrystudent.com
Benzene Structure (ALevel) ChemistryStudent What Is The Meaning Of Delocalised For example, bonding electrons may be distributed among several atoms. Localized electrons are found between atoms and are confined. delocalization happens when electric charge is spread over more than one atom. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles. What Is The Meaning Of Delocalised.
From www.youtube.com
Delocalized Electrons and Charges YouTube What Is The Meaning Of Delocalised For example, bonding electrons may be distributed among several atoms. Localized electrons are found between atoms and are confined. a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. delocalisation of an electron occurs when the valence electron of an atom does not stay in. What Is The Meaning Of Delocalised.
From alevelchemistry.co.uk
Delocalisation Facts, Summary & Definition Chemistry Revision What Is The Meaning Of Delocalised a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. delocalized electrons are not associated with any particular atom. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn. What Is The Meaning Of Delocalised.
From www.chemistrystudent.com
Metallic Bonding (ALevel) ChemistryStudent What Is The Meaning Of Delocalised Localized electrons are found between atoms and are confined. That is, the orbitals spread over the entire molecule. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles of electron movement used in writing resonance structures in lewis notation, known as the. What Is The Meaning Of Delocalised.
From www.breakingatom.com
Delocalised electrons Definition What Is The Meaning Of Delocalised a delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. That is, the orbitals spread over the entire molecule. to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the. What Is The Meaning Of Delocalised.
From www.studocu.com
Lecture 17 delocalised bonding Delocalisedbonding es f pre 7 re What Is The Meaning Of Delocalised to introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles of electron movement used in writing resonance structures in lewis notation, known as the curved arrow formalism. delocalisation of an electron occurs when the valence electron of an atom does not. What Is The Meaning Of Delocalised.
From www.pinterest.com
Delocalized electron definition and examples. Chemistry lessons What Is The Meaning Of Delocalised delocalisation of an electron occurs when the valence electron of an atom does not stay in its respective shell and starts to move around freely in valence shells of its covalently bonded molecule. For example, bonding electrons may be distributed among several atoms. Localized electrons are found between atoms and are confined. That is, the orbitals spread over the. What Is The Meaning Of Delocalised.