Copper Electroplating Half Reactions . For the example above, these would. A piece of copper is connected to the positive. The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. The participating electrode effect can be put to good use in the purification. (i) the negative cathode reaction with. It can be instructive to. This is summarised in the following table: Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. The reaction is the reverse of the cathode reaction. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper.
from wisc.pb.unizin.org
A piece of copper is connected to the positive. For the example above, these would. The participating electrode effect can be put to good use in the purification. Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. This is summarised in the following table: It can be instructive to. The reaction is the reverse of the cathode reaction. The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. (i) the negative cathode reaction with.
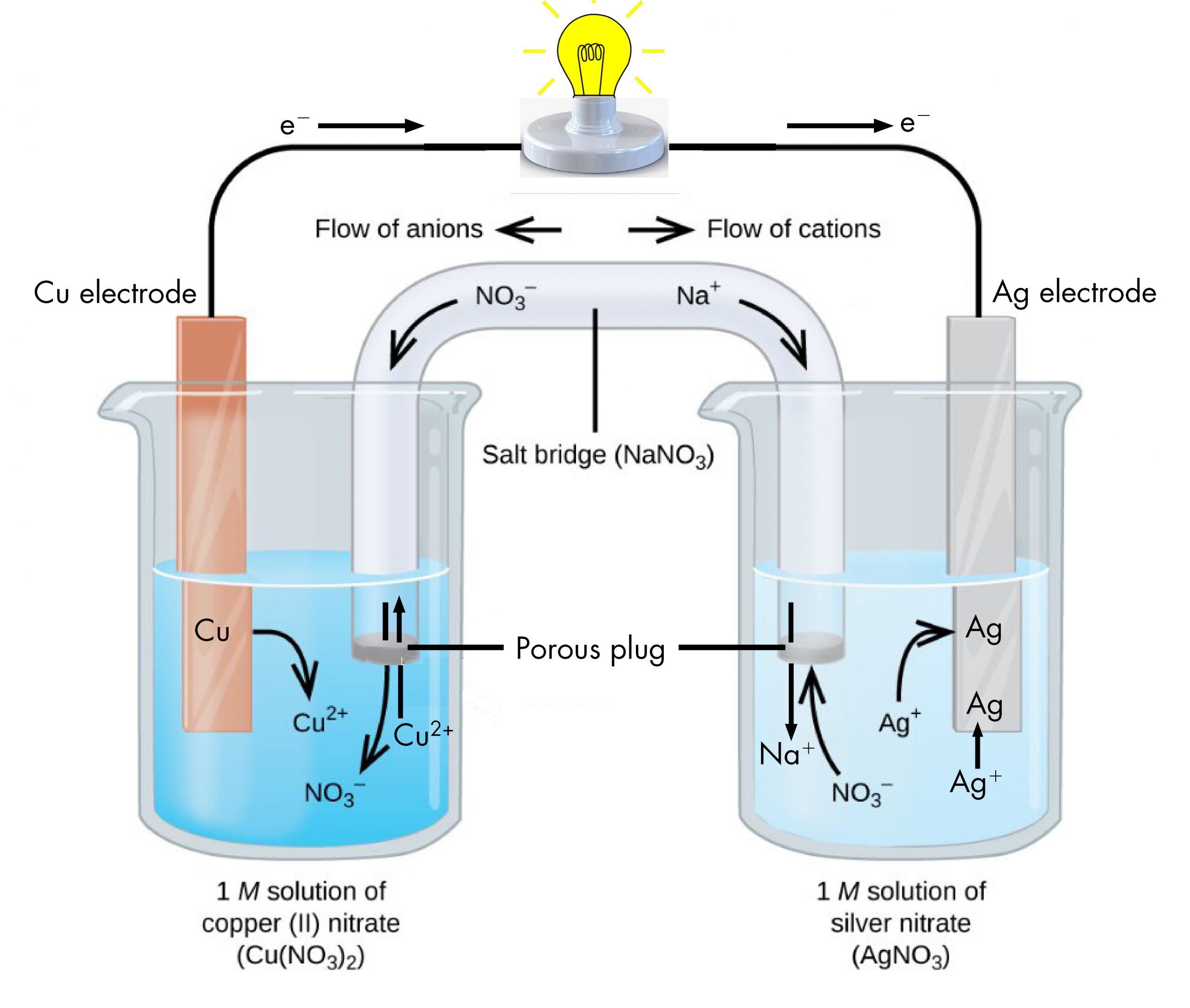
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109
Copper Electroplating Half Reactions The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. The reaction is the reverse of the cathode reaction. For the example above, these would. This is summarised in the following table: The participating electrode effect can be put to good use in the purification. (i) the negative cathode reaction with. Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. A piece of copper is connected to the positive. It can be instructive to. The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply.
From www.pinterest.com
Electroplating Part 2 Science SciencewithTylerDeWitt TylerDeWitt Copper Electroplating Half Reactions This is summarised in the following table: The participating electrode effect can be put to good use in the purification. The reaction is the reverse of the cathode reaction. Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. For. Copper Electroplating Half Reactions.
From brainly.in
[Solved] Explain electrolytic refining of copper Brainly.in Copper Electroplating Half Reactions The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. (i) the negative cathode reaction with. For the example above, these would. Oxidation/reduction reactions are often studied by running the reactions as. Copper Electroplating Half Reactions.
From www.shutterstock.com
Electroplating Copper Using Copper Sulfate Electrolyte Stock Vector Copper Electroplating Half Reactions The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. A piece of copper is connected to the positive. For the example above, these would. This is summarised in the following table:. Copper Electroplating Half Reactions.
From informacionpublica.svet.gob.gt
What Is The Chemical Equation For Copper Electroplating Copper Electroplating Half Reactions The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. It can be instructive to. The reaction is the reverse of the cathode reaction. Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. The participating electrode effect can be put to good use in the purification. Extended. Copper Electroplating Half Reactions.
From courses.lumenlearning.com
Electrolysis Chemistry Copper Electroplating Half Reactions The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. It can be instructive to. A piece of copper is connected to the positive. (i) the negative cathode reaction with. The participating electrode effect can be put to good use in the purification. The reaction is the reverse of the. Copper Electroplating Half Reactions.
From www.nagwa.com
Question Video Identifying the Setup Appropriate for Electroplating Copper Electroplating Half Reactions The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. This is summarised in the following table: (i) the negative cathode reaction with. A piece of copper is connected to the positive. The reaction is the reverse of the cathode reaction. Extended tier students may be asked to write the ionic half. Copper Electroplating Half Reactions.
From saylordotorg.github.io
Electrochemistry Copper Electroplating Half Reactions This is summarised in the following table: The participating electrode effect can be put to good use in the purification. The reaction is the reverse of the cathode reaction. (i) the negative cathode reaction with. A piece of copper is connected to the positive. The object to be plated, such as a metal pan, is connected to the negative terminal. Copper Electroplating Half Reactions.
From ar.inspiredpencil.com
Electroplating Copper Copper Electroplating Half Reactions For the example above, these would. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. A piece of copper is connected to the positive. The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. It can be instructive to. Extended tier. Copper Electroplating Half Reactions.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 Copper Electroplating Half Reactions A piece of copper is connected to the positive. Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. This is summarised in the following table: (i) the negative cathode reaction with. Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. The results of this experiment can lead to. Copper Electroplating Half Reactions.
From www.askiitians.com
Daniell Cell Study Material for IIT JEE askIITians Copper Electroplating Half Reactions Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. (i) the negative cathode reaction with. Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. This is summarised in the following table: The participating electrode effect can be put to good use in the purification. The results of this. Copper Electroplating Half Reactions.
From ytsab.mozello.com
ytsab What Is Electroplating Copper Electroplating Half Reactions Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. The participating electrode effect can be put to good use in the purification. Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. The object to be plated, such as a metal pan, is connected to the negative terminal of. Copper Electroplating Half Reactions.
From www.slideserve.com
PPT Electroplating PowerPoint Presentation, free download ID1994006 Copper Electroplating Half Reactions Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. The participating electrode effect can be put to good use in the purification. Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of. Copper Electroplating Half Reactions.
From informacionpublica.svet.gob.gt
What Is The Chemical Equation For Copper Electroplating Copper Electroplating Half Reactions The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. The reaction is the reverse of the cathode reaction. Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. This. Copper Electroplating Half Reactions.
From ar.inspiredpencil.com
Electroplating Diagram Copper Electroplating Half Reactions This is summarised in the following table: The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. It can be instructive to. A piece of copper is connected to the positive. Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. The object to be plated, such as a metal. Copper Electroplating Half Reactions.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte Copper Electroplating Half Reactions The reaction is the reverse of the cathode reaction. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. It can be instructive to. Extended tier students may be asked to write. Copper Electroplating Half Reactions.
From www.youtube.com
Introduction to Electroplating Electrochemistry YouTube Copper Electroplating Half Reactions The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. This is summarised in the following table: Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. A piece of copper is connected to the positive. Oxidation/reduction reactions are often studied by. Copper Electroplating Half Reactions.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Copper Electroplating Half Reactions Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. The reaction is the reverse of the cathode reaction. This is summarised in the following table: (i) the negative cathode reaction with. It can be instructive to. A piece of copper is connected to the positive. The object to be plated, such. Copper Electroplating Half Reactions.
From www.peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Copper Electroplating Half Reactions The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. It can be instructive to. The participating electrode effect can be put to good use in the purification. (i) the negative cathode reaction with. A piece of copper is connected to the positive. For the example above, these would. This is summarised. Copper Electroplating Half Reactions.
From byjus.com
Electrolysis of cuso4 solution using copper as electrode Copper Electroplating Half Reactions The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. (i) the negative cathode reaction with. A piece of copper is connected to the positive. The reaction is the reverse of the cathode reaction. Extended tier students may be asked to write the ionic half equations for the reaction at. Copper Electroplating Half Reactions.
From wiringfixpathway.z21.web.core.windows.net
Electric Plating Copper Diagram Copper Electroplating Half Reactions This is summarised in the following table: Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. A piece of copper is connected to the positive. The reaction is the reverse of the cathode reaction. The object to be plated,. Copper Electroplating Half Reactions.
From www.youtube.com
Electroplating & The Purification Of Copper (GCSE Chemistry) YouTube Copper Electroplating Half Reactions Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. The reaction is the reverse of the cathode reaction. For the example above, these would. Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. It can be instructive to. A piece of copper is connected to the positive. The. Copper Electroplating Half Reactions.
From www.youtube.com
What is Electroplating? Copper Electroplating YouTube Copper Electroplating Half Reactions Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. For the example above, these would. (i) the negative cathode reaction with. The participating electrode effect can be put to good use in the purification. A piece of copper is connected to the positive. This is summarised in the following table: It. Copper Electroplating Half Reactions.
From www.aakash.ac.in
Redox Reactions Definition, Types, Applications & Uses Chemistry Copper Electroplating Half Reactions The participating electrode effect can be put to good use in the purification. This is summarised in the following table: It can be instructive to. (i) the negative cathode reaction with. Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. A piece of copper is connected to the positive. The object. Copper Electroplating Half Reactions.
From anthropology.iresearchnet.com
Conductive of copper electrode working electrode PH Meters Analyzers Copper Electroplating Half Reactions The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. For the example above, these would. A piece of copper is connected to the positive. It can be instructive to. (i) the negative cathode reaction with. The participating electrode effect can be put to good use in the purification. The. Copper Electroplating Half Reactions.
From ceg.edu.vn
Share more than 138 electroplating iron nail with copper ceg.edu.vn Copper Electroplating Half Reactions The reaction is the reverse of the cathode reaction. It can be instructive to. For the example above, these would. Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. This is summarised in the following table: A piece of copper is connected to the positive. (i) the negative cathode reaction with. Extended tier students may be asked. Copper Electroplating Half Reactions.
From www.researchgate.net
The reaction principle of copper electroplating. Download Scientific Copper Electroplating Half Reactions The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. The reaction is the reverse of the cathode reaction. A piece of copper is connected to the positive. It can be instructive to. The. Copper Electroplating Half Reactions.
From brainly.in
draw the diagram showing the electrolytic refining of copper Brainly.in Copper Electroplating Half Reactions (i) the negative cathode reaction with. The reaction is the reverse of the cathode reaction. The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. It can be instructive to. Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. This is. Copper Electroplating Half Reactions.
From www.schuettemetals.com
The Marvelous Alchemy of Electroplating Copper Electroplating Half Reactions The reaction is the reverse of the cathode reaction. It can be instructive to. (i) the negative cathode reaction with. Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. A piece of copper. Copper Electroplating Half Reactions.
From www.youtube.com
Electroplating a key with copper The Real Chemist YouTube Copper Electroplating Half Reactions Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. The participating electrode effect can be put to good use in the purification. The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. (i) the negative cathode reaction with. For the example. Copper Electroplating Half Reactions.
From www.youtube.com
Electroplating CopperPlate a Key YouTube Copper Electroplating Half Reactions This is summarised in the following table: It can be instructive to. A piece of copper is connected to the positive. The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. The reaction is the reverse of the cathode reaction. Extended tier students may be asked to write the ionic. Copper Electroplating Half Reactions.
From www.youtube.com
Electrochemistry Electrolysis, Electrolytic Cell & Electroplating Copper Electroplating Half Reactions (i) the negative cathode reaction with. It can be instructive to. The reaction is the reverse of the cathode reaction. This is summarised in the following table: For the example above, these would. The participating electrode effect can be put to good use in the purification. The results of this experiment can lead to a discussion about electroplating and the. Copper Electroplating Half Reactions.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Copper Electroplating Half Reactions For the example above, these would. The participating electrode effect can be put to good use in the purification. This is summarised in the following table: A piece of copper is connected to the positive. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. The reaction is the reverse of the. Copper Electroplating Half Reactions.
From www.chemedx.org
An Easy Copper Electroplating Demo for Your Redox Unit Chemical Copper Electroplating Half Reactions For the example above, these would. A piece of copper is connected to the positive. The participating electrode effect can be put to good use in the purification. Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. The reaction is the reverse of the cathode reaction. Extended tier students may be asked to write the ionic half. Copper Electroplating Half Reactions.
From www.youtube.com
BASICS OF ELECTROPLATING OF SILVER OVER COPPER AND REDOX REACTIONS Copper Electroplating Half Reactions (i) the negative cathode reaction with. Extended tier students may be asked to write the ionic half equations for the reaction at each electrode. This is summarised in the following table: It can be instructive to. The participating electrode effect can be put to good use in the purification. Oxidation/reduction reactions are often studied by running the reactions as electrochemical. Copper Electroplating Half Reactions.
From mantavya.com
What Is Electroplating & How does it work 2021 Guide Mantavya Copper Electroplating Half Reactions The reaction is the reverse of the cathode reaction. This is summarised in the following table: A piece of copper is connected to the positive. For the example above, these would. Oxidation/reduction reactions are often studied by running the reactions as electrochemical cells. The participating electrode effect can be put to good use in the purification. (i) the negative cathode. Copper Electroplating Half Reactions.