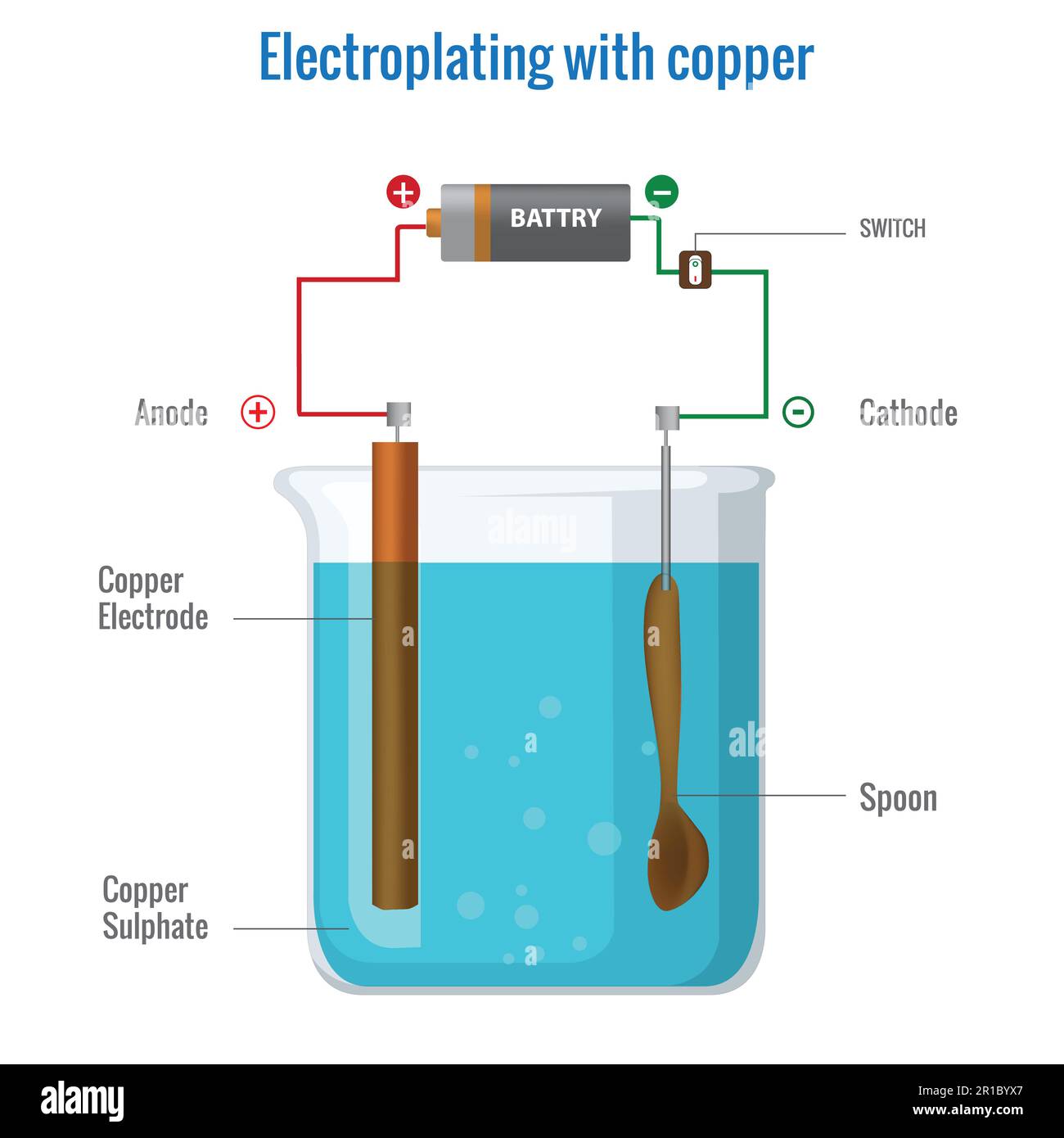
Copper Sulfate Electroplating . Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through the. The reaction is the reverse of the cathode reaction. An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper, such as copper sulfate, copper chloride, or copper cyanide. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. Alternatively, anodes and cathodes of other metals can be used in a copper sulfate solution to take copper from the solution and plate. There are two main ways to electroplate an object with copper. Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. These solutions often have an acidic compound like sulfuric acid, which helps increase the bath’s conductivity and enhances the plating rate. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help. It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability. Learn how copper electroplating works and how it is used in semiconductor packaging applications such as dual.
from www.alamy.com
These solutions often have an acidic compound like sulfuric acid, which helps increase the bath’s conductivity and enhances the plating rate. Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help. Alternatively, anodes and cathodes of other metals can be used in a copper sulfate solution to take copper from the solution and plate. It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability. Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through the. An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper, such as copper sulfate, copper chloride, or copper cyanide. The reaction is the reverse of the cathode reaction. Learn how copper electroplating works and how it is used in semiconductor packaging applications such as dual. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper.
Electroplating with copper using copper sulfate electrolyte
Copper Sulfate Electroplating Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through the. Alternatively, anodes and cathodes of other metals can be used in a copper sulfate solution to take copper from the solution and plate. Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through the. Learn how copper electroplating works and how it is used in semiconductor packaging applications such as dual. The reaction is the reverse of the cathode reaction. An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper, such as copper sulfate, copper chloride, or copper cyanide. Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. These solutions often have an acidic compound like sulfuric acid, which helps increase the bath’s conductivity and enhances the plating rate. There are two main ways to electroplate an object with copper. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help. It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte Copper Sulfate Electroplating Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. Learn how copper electroplating works and how it is used in semiconductor packaging applications such as dual. Alternatively, anodes and cathodes of other metals can be used in a copper sulfate solution to take copper from the solution and plate. These solutions. Copper Sulfate Electroplating.
From www.mdpi.com
Effect of Copper Sulfate and Sulfuric Acid on Blind Hole Filling of HDI Copper Sulfate Electroplating Learn how copper electroplating works and how it is used in semiconductor packaging applications such as dual. Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through the. These solutions often have an acidic compound like sulfuric acid, which helps increase the bath’s conductivity and enhances the plating rate. It can be. Copper Sulfate Electroplating.
From www.thoughtco.com
What Is Electroplating and How Does It Work? Copper Sulfate Electroplating An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper, such as copper sulfate, copper chloride, or copper cyanide. Learn how copper electroplating works and how it is used in semiconductor packaging applications such as dual. There are two main ways to electroplate an object with copper. Copper electroplating is a simple electrochemical process. Copper Sulfate Electroplating.
From sjzts18.en.made-in-china.com
Copper Sulphate for Feed / Agriculture / Electroplating China Copper Copper Sulfate Electroplating Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. Alternatively, anodes and cathodes of other metals can be used in a copper sulfate solution to take copper from the solution and plate. It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for. Copper Sulfate Electroplating.
From www.dreamstime.com
Electrolysis of Copper Sulfate Solution with Impure Copper Anode and Copper Sulfate Electroplating Alternatively, anodes and cathodes of other metals can be used in a copper sulfate solution to take copper from the solution and plate. These solutions often have an acidic compound like sulfuric acid, which helps increase the bath’s conductivity and enhances the plating rate. An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper,. Copper Sulfate Electroplating.
From ar.inspiredpencil.com
Aqueous Copper Sulfate Copper Sulfate Electroplating These solutions often have an acidic compound like sulfuric acid, which helps increase the bath’s conductivity and enhances the plating rate. Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. Alternatively, anodes and cathodes of other metals can be used in a copper sulfate solution to take copper from the solution. Copper Sulfate Electroplating.
From www.dreamstime.com
Electrolysis of Copper Sulfate Solution with Impure Copper Anode and Copper Sulfate Electroplating Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help. These solutions often have an acidic compound like sulfuric acid, which helps increase the bath’s conductivity and enhances the plating rate. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. Learn. Copper Sulfate Electroplating.
From www.askiitians.com
Applications Of Electrolysis Study Material for IIT JEE askIITians Copper Sulfate Electroplating Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. Learn how copper electroplating works and how it is used in semiconductor packaging applications such as dual. The reaction is the reverse of the. Copper Sulfate Electroplating.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode Copper Sulfate Electroplating Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through the. Learn how copper electroplating works and how it is used in semiconductor packaging applications such as dual. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. Alternatively, anodes and cathodes of other. Copper Sulfate Electroplating.
From byjus.com
Observe the working electroplating setup and select the correct Copper Sulfate Electroplating It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through the. Learn how. Copper Sulfate Electroplating.
From www.vrogue.co
Draw Well Labelled Diagram For The Electroplating Of vrogue.co Copper Sulfate Electroplating It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability. An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper, such as copper sulfate, copper chloride, or copper cyanide. There are two main ways to electroplate an object with copper. The reaction is. Copper Sulfate Electroplating.
From mantavya.com
What Is Electroplating & How does it work 2021 Guide Mantavya Copper Sulfate Electroplating Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help. The reaction is the reverse of the cathode reaction. The results of this experiment can lead to a discussion about electroplating and. Copper Sulfate Electroplating.
From www.youtube.com
Electrolysis Of Copper(ii) Sulphate Using Copper Electrodes YouTube Copper Sulfate Electroplating Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help. There are two main ways to electroplate an object with copper. An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper, such as copper sulfate, copper chloride, or copper cyanide. Alternatively, anodes and cathodes. Copper Sulfate Electroplating.
From www.evuchina.com
Treatment Methods for Electroplating Wastewater Copper Sulfate Electroplating These solutions often have an acidic compound like sulfuric acid, which helps increase the bath’s conductivity and enhances the plating rate. The reaction is the reverse of the cathode reaction. An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper, such as copper sulfate, copper chloride, or copper cyanide. It can be instructive to. Copper Sulfate Electroplating.
From www.verywellhealth.com
Copper Sulfate Benefits, Side Effects, Dosage, and Interactions Copper Sulfate Electroplating Learn how copper electroplating works and how it is used in semiconductor packaging applications such as dual. It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability. There are two main ways to electroplate an object with copper. The results of this experiment can lead to a discussion about. Copper Sulfate Electroplating.
From www.etsy.com
Copper Sulfate Crystals 99.8 PURE MIN. 4 X 1lb Bottles Etsy Copper Sulfate Electroplating Learn how copper electroplating works and how it is used in semiconductor packaging applications such as dual. Alternatively, anodes and cathodes of other metals can be used in a copper sulfate solution to take copper from the solution and plate. Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. An object. Copper Sulfate Electroplating.
From cartoondealer.com
Electroplating With Copper Using Copper Sulfate Electrolyte Vector Copper Sulfate Electroplating These solutions often have an acidic compound like sulfuric acid, which helps increase the bath’s conductivity and enhances the plating rate. It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability. Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through. Copper Sulfate Electroplating.
From www.chemedx.org
An Easy Copper Electroplating Demo for Your Redox Unit Chemical Copper Sulfate Electroplating The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. There are two main ways to electroplate an object with copper. It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability. Copper electroplating is a simple electrochemical process that results in. Copper Sulfate Electroplating.
From www.youtube.com
Electroplating CopperPlate a Key YouTube Copper Sulfate Electroplating The reaction is the reverse of the cathode reaction. An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper, such as copper sulfate, copper chloride, or copper cyanide. There are two main ways to electroplate an object with copper. These solutions often have an acidic compound like sulfuric acid, which helps increase the bath’s. Copper Sulfate Electroplating.
From www.alibaba.com
Electroplating Copper Sulphate Cuso4.5h2o Purity 99.5 Buy Copper Sulfate Electroplating An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper, such as copper sulfate, copper chloride, or copper cyanide. It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability. Alternatively, anodes and cathodes of other metals can be used in a copper sulfate. Copper Sulfate Electroplating.
From www.jzjcschem.com
China Top Suppliers Cu2so4 5h2o Electroplating Grade Copper Sulfate Copper Sulfate Electroplating Alternatively, anodes and cathodes of other metals can be used in a copper sulfate solution to take copper from the solution and plate. Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through the.. Copper Sulfate Electroplating.
From www.numerade.com
SOLVED Question 3 (10 points) Cus04 A student desired to electroplate Copper Sulfate Electroplating Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. The reaction is the reverse of the cathode reaction. These solutions often have an acidic compound like sulfuric acid, which helps increase the bath’s conductivity and enhances the plating rate. Learn how copper electroplating works and how it is used in semiconductor. Copper Sulfate Electroplating.
From fphoto.photoshelter.com
science chemistry electrolysis copper sulfate Fundamental Photographs Copper Sulfate Electroplating An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper, such as copper sulfate, copper chloride, or copper cyanide. There are two main ways to electroplate an object with copper. Learn how copper electroplating works and how it is used in semiconductor packaging applications such as dual. Copper electroplating is a simple electrochemical process. Copper Sulfate Electroplating.
From www.indiamart.com
Electroplating Grade Copper Sulphate at Rs 260/kg Copper Sulfate in Copper Sulfate Electroplating Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. The reaction is the reverse of the cathode reaction. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help. There are two main ways to electroplate an object with copper. Learn how. Copper Sulfate Electroplating.
From ar.inspiredpencil.com
Electroplating Copper Copper Sulfate Electroplating These solutions often have an acidic compound like sulfuric acid, which helps increase the bath’s conductivity and enhances the plating rate. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through the. Alternatively, anodes. Copper Sulfate Electroplating.
From www.youtube.com
Easy StepbyStep Tutorial on Electroplating a CopperPlated Key YouTube Copper Sulfate Electroplating Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help. Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through the. An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper, such as copper sulfate, copper. Copper Sulfate Electroplating.
From www.jzjcschem.com
China Top Suppliers Cu2so4 5h2o Electroplating Grade Copper Sulfate Copper Sulfate Electroplating Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through the. There are two main ways to electroplate an object with copper. It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability. The reaction is the reverse of the cathode reaction.. Copper Sulfate Electroplating.
From www.nagwa.com
Question Video Identifying the Setup Appropriate for Electroplating Copper Sulfate Electroplating Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help. It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability. The reaction is the reverse of the cathode reaction. Learn how copper electroplating works and how it is used. Copper Sulfate Electroplating.
From www.schuettemetals.com
The Marvelous Alchemy of Electroplating Copper Sulfate Electroplating Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help. There are two main ways to electroplate an object with copper. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. Copper electroplating is a straightforward electrochemical method that yields a thin. Copper Sulfate Electroplating.
From wjweishida.en.made-in-china.com
Electroplating Grade Copper Sulfate China Copper Sulfate and copper Copper Sulfate Electroplating An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper, such as copper sulfate, copper chloride, or copper cyanide. Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface. Copper Sulfate Electroplating.
From xtdktech.en.made-in-china.com
Copper Sulfate for Feed / Agriculture / Electroplating / Fertilizer Copper Sulfate Electroplating There are two main ways to electroplate an object with copper. Alternatively, anodes and cathodes of other metals can be used in a copper sulfate solution to take copper from the solution and plate. An object plated with copper sits in an electrolyte bath with a solution containing dissolved copper, such as copper sulfate, copper chloride, or copper cyanide. Learn. Copper Sulfate Electroplating.
From byjus.com
Electrolysis of cuso4 solution using copper as electrode Copper Sulfate Electroplating Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help. It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability. The reaction is the reverse of the cathode reaction. Alternatively, anodes and cathodes of other metals can be used. Copper Sulfate Electroplating.
From www.dreamstime.com
Electroplating with Copper Using Copper Sulfate Electrolyte Stock Copper Sulfate Electroplating The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. Learn how to electroplate copper onto a brass key using a battery, copper strip, and copper sulfate solution. Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through the. There are two main ways. Copper Sulfate Electroplating.
From www.indiamart.com
Electroplating Copper Sulphate Powder at Rs 300/kg in Gurgaon ID Copper Sulfate Electroplating It can be instructive to allow students to copperplate metal objects supplied by the school and previously tested for their suitability. The results of this experiment can lead to a discussion about electroplating and the electrolytic refining of copper. Copper electroplating is a straightforward electrochemical method that yields a thin copper coating on any conductive surface through the. Copper electroplating. Copper Sulfate Electroplating.
From www.indiamart.com
Electroplating Grade Copper Sulphate, Crystal at Rs 350/kg in Coimbatore Copper Sulfate Electroplating Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help. The reaction is the reverse of the cathode reaction. These solutions often have an acidic compound like sulfuric acid, which helps increase the bath’s conductivity and enhances the plating rate. Alternatively, anodes and cathodes of other metals can be used. Copper Sulfate Electroplating.