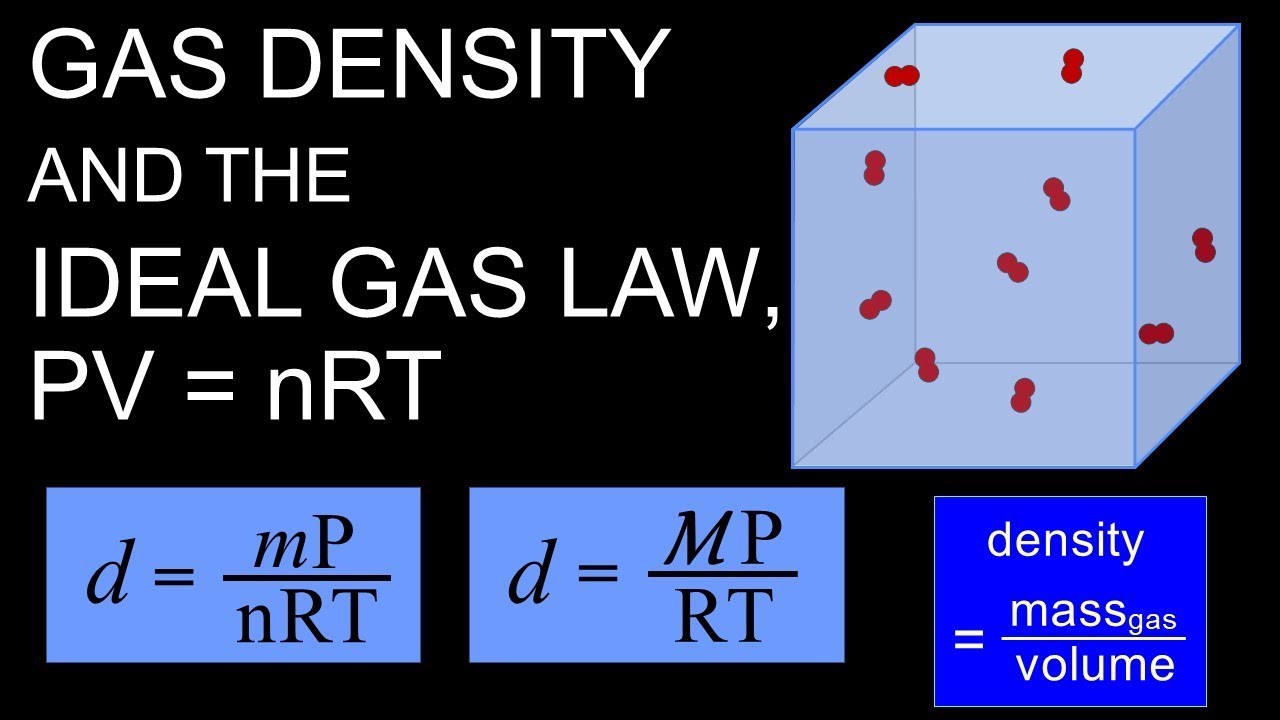
Ideal Gas Equation With Density . In a perfect or ideal. The ideal gas equation is: How to find the density of gas with the ideal gas law. On the whole, this is an easy equation to remember and use. The relationship between volume, pressure, temperature and quantity of a gas, including definition of gas density. The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles of gas molecules, r is the universal. With gas, as the volume goes up, the density goes down, and. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. You typically need to know the mass and volume to find the density.
from www.youtube.com
The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles of gas molecules, r is the universal. You typically need to know the mass and volume to find the density. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. How to find the density of gas with the ideal gas law. In a perfect or ideal. On the whole, this is an easy equation to remember and use. With gas, as the volume goes up, the density goes down, and. The relationship between volume, pressure, temperature and quantity of a gas, including definition of gas density. The ideal gas equation is:
Gas density and PV=nRT, the ideal gas law YouTube
Ideal Gas Equation With Density In a perfect or ideal. How to find the density of gas with the ideal gas law. The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. With gas, as the volume goes up, the density goes down, and. The relationship between volume, pressure, temperature and quantity of a gas, including definition of gas density. On the whole, this is an easy equation to remember and use. In a perfect or ideal. The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles of gas molecules, r is the universal. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. You typically need to know the mass and volume to find the density. The ideal gas equation is:
From inspiritvr.com
Ideal Gas Law Study Guide Inspirit Learning Inc Ideal Gas Equation With Density The ideal gas equation is: The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. You. Ideal Gas Equation With Density.
From www.coursehero.com
[Solved] What is the value of for an Ideal Gas. Express your result in Ideal Gas Equation With Density In a perfect or ideal. The relationship between volume, pressure, temperature and quantity of a gas, including definition of gas density. You typically need to know the mass and volume to find the density. The ideal gas equation is: With gas, as the volume goes up, the density goes down, and. The ideal gas law equation is pv = nrt,. Ideal Gas Equation With Density.
From www.slideserve.com
PPT Gas Law Calculations PowerPoint Presentation, free download ID Ideal Gas Equation With Density With gas, as the volume goes up, the density goes down, and. How to find the density of gas with the ideal gas law. The ideal gas equation is: The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. On the whole, this is an easy. Ideal Gas Equation With Density.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID6591194 Ideal Gas Equation With Density How to find the density of gas with the ideal gas law. In a perfect or ideal. The relationship between volume, pressure, temperature and quantity of a gas, including definition of gas density. On the whole, this is an easy equation to remember and use. The ideal gas law is derived from empirical relationships among the pressure, the volume, the. Ideal Gas Equation With Density.
From byjus.com
Different forms of ideal gas equation Ideal Gas Equation With Density The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles of gas molecules, r is the universal. How to find the density. Ideal Gas Equation With Density.
From www.slideserve.com
PPT Gas Density PowerPoint Presentation, free download ID3286907 Ideal Gas Equation With Density The ideal gas equation is: In a perfect or ideal. On the whole, this is an easy equation to remember and use. How to find the density of gas with the ideal gas law. The relationship between volume, pressure, temperature and quantity of a gas, including definition of gas density. The ideal gas law equation is pv = nrt, where. Ideal Gas Equation With Density.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Ideal Gas Equation With Density In a perfect or ideal. With gas, as the volume goes up, the density goes down, and. The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. How to find the density of gas with the ideal gas law. The. Ideal Gas Equation With Density.
From www.toppr.com
Derive the relationship between pressure, temperature, and density of a Ideal Gas Equation With Density How to find the density of gas with the ideal gas law. The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles of gas molecules, r is the universal. The ideal gas equation is: On the whole, this is an easy equation to remember and use.. Ideal Gas Equation With Density.
From thtcsqpcfj.blogspot.com
How To Find The Density Of Something Plug your values into the Ideal Gas Equation With Density The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. In a perfect or ideal. The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. The. Ideal Gas Equation With Density.
From slideplayer.com
States of Matter Lesson ppt download Ideal Gas Equation With Density With gas, as the volume goes up, the density goes down, and. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles. Ideal Gas Equation With Density.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Ideal Gas Equation With Density In a perfect or ideal. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. How to find the density of gas with the ideal gas law. The ideal gas equation is: On the whole, this is an easy equation to remember and use. The relationship. Ideal Gas Equation With Density.
From www.slideserve.com
PPT The Ideal Gas Law 01 PowerPoint Presentation, free download ID Ideal Gas Equation With Density With gas, as the volume goes up, the density goes down, and. You typically need to know the mass and volume to find the density. The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. The relationship between volume, pressure,. Ideal Gas Equation With Density.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Ideal Gas Equation With Density In a perfect or ideal. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. You typically need to know the mass and volume to find the density. The relationship between volume, pressure, temperature and quantity of a gas, including definition of gas density. On the. Ideal Gas Equation With Density.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii Ideal Gas Equation With Density The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. How to find the density of gas with the ideal gas law. The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume,. Ideal Gas Equation With Density.
From www.grc.nasa.gov
Equation of State Ideal Gas Equation With Density The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. With gas, as the volume goes. Ideal Gas Equation With Density.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID4199554 Ideal Gas Equation With Density The ideal gas equation is: The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles of. Ideal Gas Equation With Density.
From www.youtube.com
Using the Ideal Gas Law to find density or molar mass YouTube Ideal Gas Equation With Density With gas, as the volume goes up, the density goes down, and. The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles of gas molecules, r is the universal. On the whole, this is an easy equation to remember and use. The ideal gas law is. Ideal Gas Equation With Density.
From www.slideserve.com
PPT Density PowerPoint Presentation, free download ID5397411 Ideal Gas Equation With Density On the whole, this is an easy equation to remember and use. How to find the density of gas with the ideal gas law. The ideal gas equation is: The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles of gas molecules, r is the universal.. Ideal Gas Equation With Density.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4144868 Ideal Gas Equation With Density The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. On the whole, this is an. Ideal Gas Equation With Density.
From slideplayer.com
States of Matter Lesson ppt download Ideal Gas Equation With Density How to find the density of gas with the ideal gas law. The ideal gas equation is: On the whole, this is an easy equation to remember and use. In a perfect or ideal. The relationship between volume, pressure, temperature and quantity of a gas, including definition of gas density. You typically need to know the mass and volume to. Ideal Gas Equation With Density.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Ideal Gas Equation With Density With gas, as the volume goes up, the density goes down, and. How to find the density of gas with the ideal gas law. The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. You typically need to know the. Ideal Gas Equation With Density.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation Ideal Gas Equation With Density You typically need to know the mass and volume to find the density. How to find the density of gas with the ideal gas law. With gas, as the volume goes up, the density goes down, and. On the whole, this is an easy equation to remember and use. The relationship between volume, pressure, temperature and quantity of a gas,. Ideal Gas Equation With Density.
From www.youtube.com
1.3 Ideal gas equation YouTube Ideal Gas Equation With Density On the whole, this is an easy equation to remember and use. The ideal gas equation is: You typically need to know the mass and volume to find the density. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. The relationship between volume, pressure, temperature. Ideal Gas Equation With Density.
From mmerevise.co.uk
The Ideal Gas Equation MME Ideal Gas Equation With Density You typically need to know the mass and volume to find the density. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of. Ideal Gas Equation With Density.
From www.meritnation.com
using the ideal gas equation pv=nrt show that density is directly Ideal Gas Equation With Density The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. With gas, as the volume goes up, the density goes down, and. The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume,. Ideal Gas Equation With Density.
From www.dailymotion.com
Ideal Gas Equation Crash Course Chemistry 12 Derivation of pv=nrt Ideal Gas Equation With Density You typically need to know the mass and volume to find the density. On the whole, this is an easy equation to remember and use. The ideal gas equation is: How to find the density of gas with the ideal gas law. In a perfect or ideal. With gas, as the volume goes up, the density goes down, and. The. Ideal Gas Equation With Density.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Ideal Gas Equation With Density In a perfect or ideal. You typically need to know the mass and volume to find the density. With gas, as the volume goes up, the density goes down, and. The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles of gas molecules, r is the. Ideal Gas Equation With Density.
From www.slideserve.com
PPT Equation of State (a.k.a. the “ Ideal Gas Law ” ) PowerPoint Ideal Gas Equation With Density You typically need to know the mass and volume to find the density. The ideal gas equation is: In a perfect or ideal. On the whole, this is an easy equation to remember and use. With gas, as the volume goes up, the density goes down, and. How to find the density of gas with the ideal gas law. The. Ideal Gas Equation With Density.
From www.youtube.com
Ideal Gas Law Density.wmv YouTube Ideal Gas Equation With Density The relationship between volume, pressure, temperature and quantity of a gas, including definition of gas density. On the whole, this is an easy equation to remember and use. In a perfect or ideal. With gas, as the volume goes up, the density goes down, and. How to find the density of gas with the ideal gas law. The ideal gas. Ideal Gas Equation With Density.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Ideal Gas Equation With Density The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. The ideal gas equation is: The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles of. Ideal Gas Equation With Density.
From www.toppr.com
What is the ideal gas equation? How can it be derived? Also, express it Ideal Gas Equation With Density How to find the density of gas with the ideal gas law. On the whole, this is an easy equation to remember and use. The ideal gas equation is: The relationship between volume, pressure, temperature and quantity of a gas, including definition of gas density. The density ρ of a sample of gas is equal to the molar mass divided. Ideal Gas Equation With Density.
From en.ppt-online.org
The ideal gas equation online presentation Ideal Gas Equation With Density You typically need to know the mass and volume to find the density. The density ρ of a sample of gas is equal to the molar mass divided by the molar volume, and hence the equation of state for an ideal gas can. In a perfect or ideal. The relationship between volume, pressure, temperature and quantity of a gas, including. Ideal Gas Equation With Density.
From www.youtube.com
Gas density and PV=nRT, the ideal gas law YouTube Ideal Gas Equation With Density The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles of gas molecules, r is the universal. How to find the density of gas with the ideal gas law. The density ρ of a sample of gas is equal to the molar mass divided by the. Ideal Gas Equation With Density.
From www.slideserve.com
PPT Thermodynamic Properties PowerPoint Presentation, free download Ideal Gas Equation With Density The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles of gas molecules, r is the universal. In a perfect or ideal. With gas, as the volume goes up, the density goes down, and. The relationship between volume, pressure, temperature and quantity of a gas, including. Ideal Gas Equation With Density.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Ideal Gas Equation With Density The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. The ideal gas law equation is pv = nrt, where p is the pressure, v is the volume, ‘n’ is the number of moles of gas molecules, r is the universal. The relationship between volume, pressure,. Ideal Gas Equation With Density.