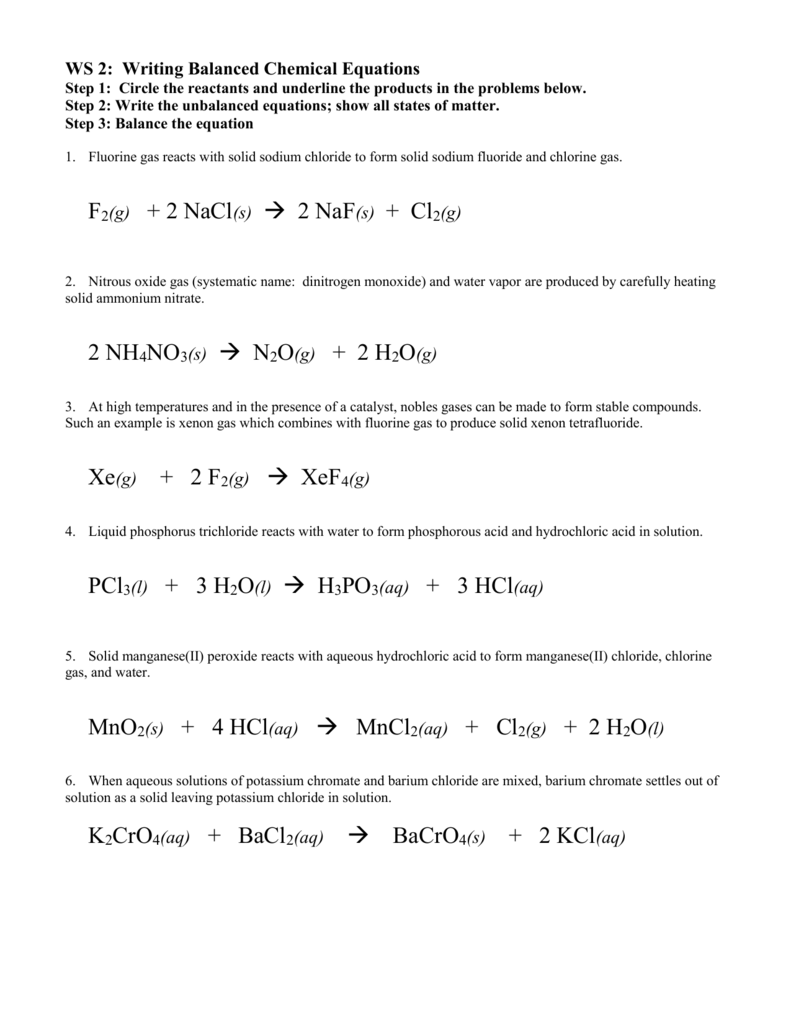
Chlorine Gas Reacts With Sodium Fluoride . 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. Sodium + chlorine → sodium chloride. Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. For example, chlorine reacts with sodium: How many liters of flurine gas are produced from the. Sodium chloride reacts with fluorine gas to produce solid sodium fluoride and chlorine gas. When sodium fluoride reacts with chlorine gas, sodium chloride and fluorine gas are produced. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. Sodium chloride react with fluorine to produce sodium fluoride and chlorine. Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. 2nacl (s) + f2 (g) → 2naf (s)+ cl2 (g) pbo2 (s) e, f.
from studylib.net
Sodium chloride react with fluorine to produce sodium fluoride and chlorine. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. 2nacl (s) + f2 (g) → 2naf (s)+ cl2 (g) pbo2 (s) e, f. When sodium fluoride reacts with chlorine gas, sodium chloride and fluorine gas are produced. Sodium chloride reacts with fluorine gas to produce solid sodium fluoride and chlorine gas. Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. For example, chlorine reacts with sodium: Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. Sodium + chlorine → sodium chloride.
WS 2 answers
Chlorine Gas Reacts With Sodium Fluoride Sodium + chlorine → sodium chloride. Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. When sodium fluoride reacts with chlorine gas, sodium chloride and fluorine gas are produced. 2nacl (s) + f2 (g) → 2naf (s)+ cl2 (g) pbo2 (s) e, f. Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. For example, chlorine reacts with sodium: Sodium chloride react with fluorine to produce sodium fluoride and chlorine. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. How many liters of flurine gas are produced from the. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. Sodium + chlorine → sodium chloride. Sodium chloride reacts with fluorine gas to produce solid sodium fluoride and chlorine gas.
From shaunmwilliams.com
Chapter 17 Presentation Chlorine Gas Reacts With Sodium Fluoride Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. 2na (s) + cl 2 (g) →. Chlorine Gas Reacts With Sodium Fluoride.
From pediaa.com
Difference Between Sodium Fluoride and Fluoride Definition Chlorine Gas Reacts With Sodium Fluoride How many liters of flurine gas are produced from the. Sodium chloride react with fluorine to produce sodium fluoride and chlorine. 2nacl (s) + f2 (g) → 2naf (s)+ cl2 (g) pbo2 (s) e, f. Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. Nacl + f = naf + cl. Chlorine Gas Reacts With Sodium Fluoride.
From edu.rsc.org
The reaction between sodium and chlorine Exhibition chemistry RSC Chlorine Gas Reacts With Sodium Fluoride Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. For example, chlorine reacts with sodium: F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two. Chlorine Gas Reacts With Sodium Fluoride.
From www.slideshare.net
Chemical Reactions Chlorine Gas Reacts With Sodium Fluoride When sodium fluoride reacts with chlorine gas, sodium chloride and fluorine gas are produced. For example, chlorine reacts with sodium: How many liters of flurine gas are produced from the. Sodium + chlorine → sodium chloride. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of. Chlorine Gas Reacts With Sodium Fluoride.
From www.youtube.com
Type of Reaction for Na + Cl2 = NaCl (Sodium + Chlorine gas) YouTube Chlorine Gas Reacts With Sodium Fluoride How many liters of flurine gas are produced from the. Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. When sodium fluoride reacts with. Chlorine Gas Reacts With Sodium Fluoride.
From www.slideserve.com
PPT CHEMICAL REACTIONS PowerPoint Presentation, free download ID142552 Chlorine Gas Reacts With Sodium Fluoride How many liters of flurine gas are produced from the. Sodium chloride react with fluorine to produce sodium fluoride and chlorine. Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. When sodium fluoride reacts with chlorine gas, sodium chloride and fluorine gas are produced. F + nacl = naf + cl2. Chlorine Gas Reacts With Sodium Fluoride.
From www.researchgate.net
A schematic showing the process of producing sodium fluoride solutions Chlorine Gas Reacts With Sodium Fluoride 2nacl (s) + f2 (g) → 2naf (s)+ cl2 (g) pbo2 (s) e, f. Sodium chloride react with fluorine to produce sodium fluoride and chlorine. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. Chlorine is a highly reactive element and would. Chlorine Gas Reacts With Sodium Fluoride.
From www.chegg.com
Solved Part A Sodium metal reacts with chlorine gas Chlorine Gas Reacts With Sodium Fluoride For example, chlorine reacts with sodium: When sodium fluoride reacts with chlorine gas, sodium chloride and fluorine gas are produced. Sodium chloride react with fluorine to produce sodium fluoride and chlorine. Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. Sodium chloride reacts. Chlorine Gas Reacts With Sodium Fluoride.
From www.numerade.com
SOLVED Sodium chloride (NaCl) reacts with fluorine gas (F2) to produce Chlorine Gas Reacts With Sodium Fluoride Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. When sodium fluoride reacts with chlorine gas, sodium chloride and fluorine gas are produced. Sodium chloride react with fluorine to produce sodium fluoride and chlorine. 2nacl (s) + f2 (g) → 2naf (s)+ cl2. Chlorine Gas Reacts With Sodium Fluoride.
From shaunmwilliams.com
Chapter 4 Presentation Chlorine Gas Reacts With Sodium Fluoride Sodium chloride react with fluorine to produce sodium fluoride and chlorine. Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. How many liters of flurine gas are produced from the. F + nacl = naf + cl2 is a single displacement (substitution) reaction. Chlorine Gas Reacts With Sodium Fluoride.
From www.chegg.com
Solved Chlorine gas reacts with fluorine gas to form Chlorine Gas Reacts With Sodium Fluoride When sodium fluoride reacts with chlorine gas, sodium chloride and fluorine gas are produced. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. For example, chlorine reacts with sodium: Sodium + chlorine → sodium chloride. Sodium chloride reacts with fluorine gas to. Chlorine Gas Reacts With Sodium Fluoride.
From stock.adobe.com
Synthesis reaction sodium chloride formation of sodium metal and Chlorine Gas Reacts With Sodium Fluoride 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. How many liters of flurine gas are produced from the. Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous. Chlorine Gas Reacts With Sodium Fluoride.
From www.numerade.com
SOLVED Ammonia gas reacts with chlorine trifluoride gas to produce Chlorine Gas Reacts With Sodium Fluoride Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. Sodium + chlorine → sodium chloride. Sodium chloride react with fluorine to produce sodium fluoride and chlorine. F + nacl. Chlorine Gas Reacts With Sodium Fluoride.
From www.grainger.com
7681494, 41.99, Sodium Fluoride, Powder, Reagent, ACS 6NNP1S1280 Chlorine Gas Reacts With Sodium Fluoride Sodium chloride react with fluorine to produce sodium fluoride and chlorine. Sodium chloride reacts with fluorine gas to produce solid sodium fluoride and chlorine gas. How many liters of flurine gas are produced from the. 2nacl (s) + f2 (g) → 2naf (s)+ cl2 (g) pbo2 (s) e, f. Sodium + chlorine → sodium chloride. Chlorine is a highly reactive. Chlorine Gas Reacts With Sodium Fluoride.
From www.coursehero.com
[Solved] Consider the reaction between sodium metal and chlorine gas to Chlorine Gas Reacts With Sodium Fluoride For example, chlorine reacts with sodium: Sodium chloride react with fluorine to produce sodium fluoride and chlorine. 2nacl (s) + f2 (g) → 2naf (s)+ cl2 (g) pbo2 (s) e, f. When sodium fluoride reacts with chlorine gas, sodium chloride and fluorine gas are produced. Sodium + chlorine → sodium chloride. F + nacl = naf + cl2 is a. Chlorine Gas Reacts With Sodium Fluoride.
From virttodo.weebly.com
Chlorines reactivity virttodo Chlorine Gas Reacts With Sodium Fluoride How many liters of flurine gas are produced from the. When sodium fluoride reacts with chlorine gas, sodium chloride and fluorine gas are produced. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. Sodium chloride reacts with fluorine gas to produce solid. Chlorine Gas Reacts With Sodium Fluoride.
From www.youtube.com
Making table salt using sodium metal and chlorine gas YouTube Chlorine Gas Reacts With Sodium Fluoride F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. For example, chlorine reacts with sodium: Sodium + chlorine → sodium chloride. Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. Sodium chloride reacts. Chlorine Gas Reacts With Sodium Fluoride.
From www.chegg.com
Solved 66. Chlorine and fluorine react to form gaseous Chlorine Gas Reacts With Sodium Fluoride Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. Sodium + chlorine → sodium chloride. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. How many liters of flurine gas are produced from the. Sodium chloride react with. Chlorine Gas Reacts With Sodium Fluoride.
From studylib.net
WS 2 answers Chlorine Gas Reacts With Sodium Fluoride How many liters of flurine gas are produced from the. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. Sodium chloride react with fluorine to produce sodium fluoride. Chlorine Gas Reacts With Sodium Fluoride.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Chlorine Gas Reacts With Sodium Fluoride F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. For example, chlorine reacts with sodium: Chlorine is a highly reactive element and would likely react with sodium fluoride. Chlorine Gas Reacts With Sodium Fluoride.
From www.chegg.com
Solved 6. Sodium metal reacts with chlorine gas to produce Chlorine Gas Reacts With Sodium Fluoride 2nacl (s) + f2 (g) → 2naf (s)+ cl2 (g) pbo2 (s) e, f. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. Sodium + chlorine → sodium chloride. Chlorine is a highly reactive element and would likely react with sodium fluoride. Chlorine Gas Reacts With Sodium Fluoride.
From www.slideserve.com
PPT Naming of Compounds PowerPoint Presentation, free download ID Chlorine Gas Reacts With Sodium Fluoride 2nacl (s) + f2 (g) → 2naf (s)+ cl2 (g) pbo2 (s) e, f. Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. Sodium + chlorine → sodium chloride. Chlorine is a highly reactive element and would likely react with sodium fluoride solution. Chlorine Gas Reacts With Sodium Fluoride.
From www.slideserve.com
PPT General Chemistry (I Chlorine Gas Reacts With Sodium Fluoride When sodium fluoride reacts with chlorine gas, sodium chloride and fluorine gas are produced. Sodium + chlorine → sodium chloride. Sodium chloride reacts with fluorine gas to produce solid sodium fluoride and chlorine gas. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. F + nacl = naf + cl2 is a single displacement (substitution). Chlorine Gas Reacts With Sodium Fluoride.
From www.doubtnut.com
When Cl(2) gas reacts with hot and concentrated sodium hydroxide solut Chlorine Gas Reacts With Sodium Fluoride Sodium + chlorine → sodium chloride. Sodium chloride reacts with fluorine gas to produce solid sodium fluoride and chlorine gas. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. Chlorine is a highly reactive element and would likely react with sodium fluoride. Chlorine Gas Reacts With Sodium Fluoride.
From www.youtube.com
Reaction of sodium metal with chlorine gas YouTube Chlorine Gas Reacts With Sodium Fluoride F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. For example, chlorine reacts with sodium: Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. Sodium chloride reacts with fluorine gas to produce solid. Chlorine Gas Reacts With Sodium Fluoride.
From www.doubtnut.com
When Cl(2) gas reacts with hot and concentrated sodium hydroxide solut Chlorine Gas Reacts With Sodium Fluoride Sodium + chlorine → sodium chloride. Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. Sodium chloride reacts with fluorine gas to produce solid sodium fluoride and chlorine gas. 2nacl (s) + f2 (g) → 2naf (s)+ cl2 (g) pbo2 (s) e, f. When sodium fluoride reacts with chlorine gas, sodium. Chlorine Gas Reacts With Sodium Fluoride.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Chlorine Gas Reacts With Sodium Fluoride Sodium chloride react with fluorine to produce sodium fluoride and chlorine. Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles. Chlorine Gas Reacts With Sodium Fluoride.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Chlorine Gas Reacts With Sodium Fluoride Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. How many liters of flurine gas are produced from the. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous. Chlorine Gas Reacts With Sodium Fluoride.
From www.numerade.com
SOLVED When heated in chlorine gas, Cl2, sodium reacts violently to Chlorine Gas Reacts With Sodium Fluoride Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. Sodium chloride react with fluorine to produce sodium fluoride and chlorine. Sodium + chlorine → sodium chloride. 2nacl (s) + f2 (g) → 2naf (s)+ cl2 (g) pbo2. Chlorine Gas Reacts With Sodium Fluoride.
From www.numerade.com
SOLVEDSodium reacts with chlorine gas according to the reaction 2 Na Chlorine Gas Reacts With Sodium Fluoride 2nacl (s) + f2 (g) → 2naf (s)+ cl2 (g) pbo2 (s) e, f. Sodium chloride react with fluorine to produce sodium fluoride and chlorine. Sodium + chlorine → sodium chloride. Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. How many liters. Chlorine Gas Reacts With Sodium Fluoride.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Chlorine Gas Reacts With Sodium Fluoride Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. Sodium + chlorine → sodium chloride. Sodium chloride react with fluorine to produce sodium fluoride and. Chlorine Gas Reacts With Sodium Fluoride.
From www.numerade.com
SOLVED Chlorine gas reacts with fluorine gas to form chlorine Chlorine Gas Reacts With Sodium Fluoride F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. Sodium chloride reacts with fluorine gas to produce solid sodium fluoride and chlorine gas. Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. 2nacl. Chlorine Gas Reacts With Sodium Fluoride.
From www.numerade.com
SOLVED Question 9 solid sodium chloride is produced by the reaction of Chlorine Gas Reacts With Sodium Fluoride Nacl + f = naf + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride [nacl] and one mole of fluorine. Chlorine is a highly reactive element and would likely react with sodium fluoride solution to form various products. For example, chlorine reacts with sodium: Sodium chloride reacts with fluorine gas to produce solid sodium. Chlorine Gas Reacts With Sodium Fluoride.
From dxoqukybg.blob.core.windows.net
Chlorine Reaction With Water Formula at Cathryn Williams blog Chlorine Gas Reacts With Sodium Fluoride How many liters of flurine gas are produced from the. Sodium chloride reacts with fluorine gas to produce solid sodium fluoride and chlorine gas. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. Sodium chloride react with fluorine to produce sodium fluoride. Chlorine Gas Reacts With Sodium Fluoride.
From www.youtube.com
How to Balance F2 + Cl2 = ClF3 (Fluorine gas + Chlorine gas) YouTube Chlorine Gas Reacts With Sodium Fluoride Sodium chloride reacts with fluorine gas to produce solid sodium fluoride and chlorine gas. Sodium + chlorine → sodium chloride. F + nacl = naf + cl2 is a single displacement (substitution) reaction where two moles of fluorine [f] gas and two moles of aqueous sodium chloride. How many liters of flurine gas are produced from the. Sodium chloride react. Chlorine Gas Reacts With Sodium Fluoride.