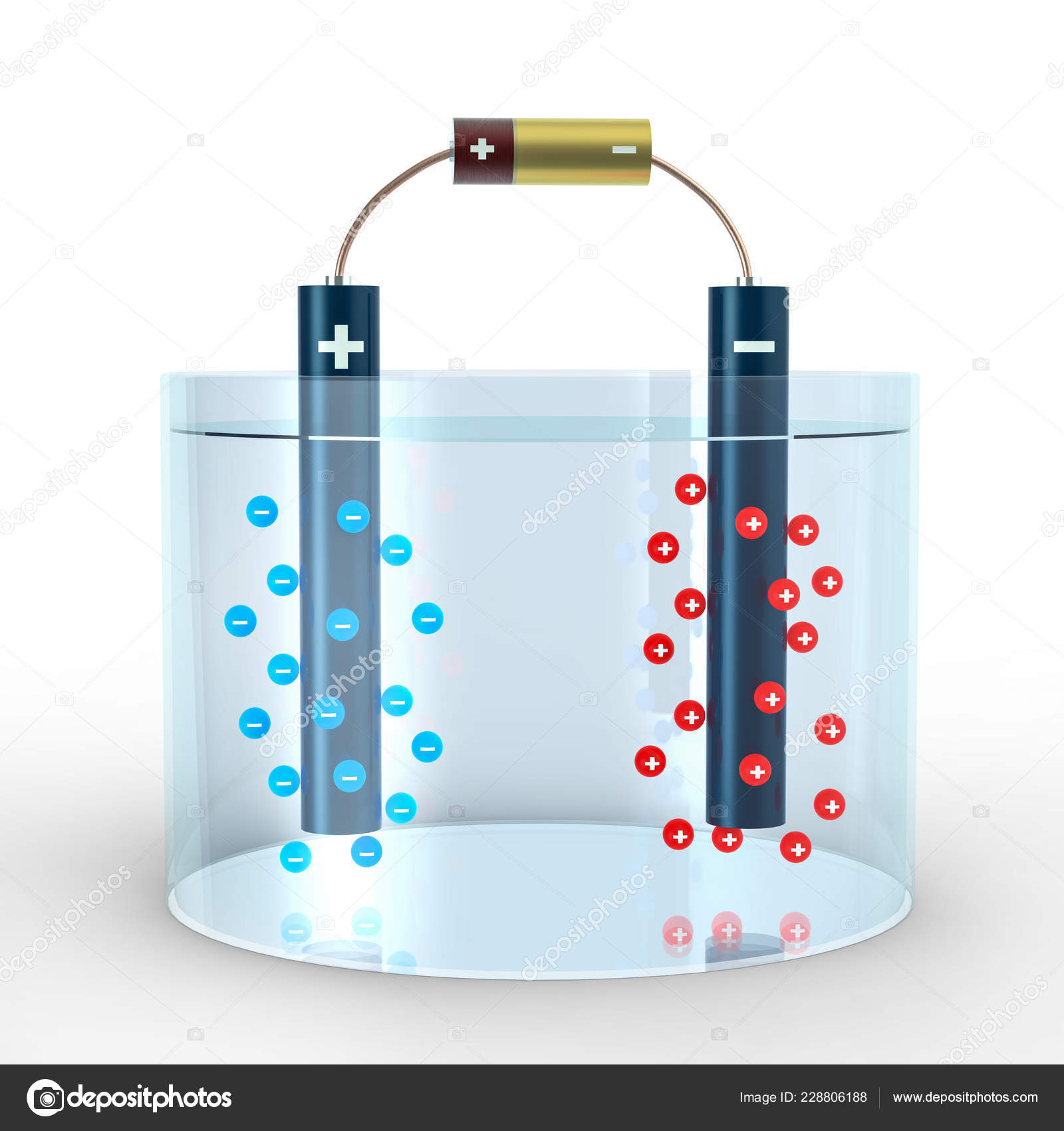
Is The Red Electrode Positive Or Negative . The anode is the negative electrode. During discharge, positive ions flow from anode to cathode. This seems reasonable as the anode. the cathode is the positive electrode; learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. in a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. The one in the tin solution is the anode. are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? the pt electrode in the permanganate solution is the cathode; in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently plate. learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron.
from virtheritage.weebly.com
learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron. in a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. The anode is the negative electrode. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently plate. The one in the tin solution is the anode. During discharge, positive ions flow from anode to cathode. are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? the pt electrode in the permanganate solution is the cathode; the cathode is the positive electrode;
Cathode negative or positive virtheritage
Is The Red Electrode Positive Or Negative in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently plate. are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron. The anode is the negative electrode. This seems reasonable as the anode. in a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. During discharge, positive ions flow from anode to cathode. The one in the tin solution is the anode. the pt electrode in the permanganate solution is the cathode; learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently plate. the cathode is the positive electrode;
From www.researchgate.net
Cell voltage (red) and negative (green) and positive electrode (black Is The Red Electrode Positive Or Negative During discharge, positive ions flow from anode to cathode. learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. The anode is the negative electrode. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently. Is The Red Electrode Positive Or Negative.
From www.vedantu.com
Cathode and Anode Definition and Difference Between Anode and Cathode Is The Red Electrode Positive Or Negative The anode is the negative electrode. The one in the tin solution is the anode. learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently. Is The Red Electrode Positive Or Negative.
From hxeledgaa.blob.core.windows.net
Electrode Regeneration Procedure at Santos blog Is The Red Electrode Positive Or Negative are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? This seems reasonable as the anode. learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron. in a galvanic cell, it acts as the positive. Is The Red Electrode Positive Or Negative.
From www.theknowledgelibrary.in
Difference Between Cation & Anion The Knowledge Library Is The Red Electrode Positive Or Negative learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. This seems reasonable as the anode. The anode is the negative electrode. During discharge, positive ions flow from anode to cathode. The one in the tin solution is the anode. the pt electrode in the permanganate solution. Is The Red Electrode Positive Or Negative.
From exoxpbgzu.blob.core.windows.net
Define Electrodes In Chemistry Terms at Monte Cordell blog Is The Red Electrode Positive Or Negative the pt electrode in the permanganate solution is the cathode; in a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. the cathode is the positive electrode; This seems reasonable as the anode. The one in the tin solution is the anode. During discharge, positive ions flow from anode to cathode. The. Is The Red Electrode Positive Or Negative.
From www.alamy.com
Electrolysis hires stock photography and images Alamy Is The Red Electrode Positive Or Negative in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently plate. in a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive. Is The Red Electrode Positive Or Negative.
From www.pinterest.co.uk
ECG. Color coding standards for 12lead ECG AHA and IEC Color coding Is The Red Electrode Positive Or Negative learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently plate. the pt electrode in the permanganate solution is the cathode; During discharge, positive. Is The Red Electrode Positive Or Negative.
From stock.adobe.com
Ions movement to negative electrode and positive electrode Stock Photo Is The Red Electrode Positive Or Negative learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. the pt electrode in the permanganate solution is the cathode; are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? The anode is the negative electrode. . Is The Red Electrode Positive Or Negative.
From www.researchgate.net
Direct connection between the red electrode ("right shoulder Is The Red Electrode Positive Or Negative in a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron. The anode is the negative electrode. learn the difference between anode and cathode, the electrodes of a cell. Is The Red Electrode Positive Or Negative.
From www.dreamstime.com
Ions Movement To Negative Electrode and Positive Electrode Stock Is The Red Electrode Positive Or Negative This seems reasonable as the anode. The anode is the negative electrode. are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. The one in the tin solution. Is The Red Electrode Positive Or Negative.
From alevelchemistry.co.uk
Redox and Electrode Potential ALevel Chemistry Revision Notes Is The Red Electrode Positive Or Negative the cathode is the positive electrode; the pt electrode in the permanganate solution is the cathode; learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. This seems reasonable as the anode. learn how to measure and interpret electrode and cell potentials, which reflect the. Is The Red Electrode Positive Or Negative.
From fixlibrarymarkbladgr.z13.web.core.windows.net
Cathode Charge In Electrolytic Cell Is The Red Electrode Positive Or Negative are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. the pt electrode in the permanganate solution is the cathode; learn how to measure and interpret. Is The Red Electrode Positive Or Negative.
From vdocuments.mx
negative electrode (anode) positive electrode (cathode ) anode cathode Is The Red Electrode Positive Or Negative During discharge, positive ions flow from anode to cathode. are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? the pt electrode in the permanganate solution is the cathode; in a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. learn the. Is The Red Electrode Positive Or Negative.
From lulidp.weebly.com
Cathode positive or negative lulidp Is The Red Electrode Positive Or Negative are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? the pt electrode in the permanganate solution is the cathode; The one in the tin solution is the anode. This seems reasonable as the anode. During discharge, positive ions flow from anode to cathode. learn how to measure. Is The Red Electrode Positive Or Negative.
From www.alamy.com
Ions movement to negative electrode and positive electrode. 3D Is The Red Electrode Positive Or Negative the pt electrode in the permanganate solution is the cathode; in a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. This seems reasonable as the anode. The anode is the. Is The Red Electrode Positive Or Negative.
From bastatrain.weebly.com
Cathode led positive or negative bastatrain Is The Red Electrode Positive Or Negative learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. During discharge, positive ions flow from anode to cathode. the pt electrode in the permanganate solution is the cathode; This seems reasonable as the anode. are the electrodes of the (zn +2 /cu) cell that is. Is The Red Electrode Positive Or Negative.
From chem.libretexts.org
14.E OxidationReduction Reaction (Exercises) Chemistry LibreTexts Is The Red Electrode Positive Or Negative This seems reasonable as the anode. the pt electrode in the permanganate solution is the cathode; learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron. The anode is the negative electrode. the cathode is the positive electrode; in a galvanic cell, it. Is The Red Electrode Positive Or Negative.
From www.iontocentre.com
Blog Red, Black Positive, Negative, Anode & Cathode? IontoCentre Is The Red Electrode Positive Or Negative learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently plate. The one in the tin solution is the anode. the pt. Is The Red Electrode Positive Or Negative.
From virtheritage.weebly.com
Cathode negative or positive virtheritage Is The Red Electrode Positive Or Negative the cathode is the positive electrode; in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently plate. The anode is the negative electrode. The one in the tin solution is the anode. are the electrodes of the (zn +2 /cu) cell that is described in. Is The Red Electrode Positive Or Negative.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID297944 Is The Red Electrode Positive Or Negative The one in the tin solution is the anode. learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? learn how to measure and interpret electrode and. Is The Red Electrode Positive Or Negative.
From giodwrxvk.blob.core.windows.net
Electrodes In Electrolysis at Lindsay Macy blog Is The Red Electrode Positive Or Negative the cathode is the positive electrode; During discharge, positive ions flow from anode to cathode. The one in the tin solution is the anode. learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron. the pt electrode in the permanganate solution is the cathode;. Is The Red Electrode Positive Or Negative.
From www.numerade.com
SOLVED 'Help!!!!!!!!!!!!!!!!!!!!! What are the names of the positive Is The Red Electrode Positive Or Negative learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron. the pt electrode in the permanganate solution is the cathode; learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. in a. Is The Red Electrode Positive Or Negative.
From www.researchgate.net
Potentials vs. NHE of positive and negative electrode vs. voltage of Is The Red Electrode Positive Or Negative This seems reasonable as the anode. The one in the tin solution is the anode. the cathode is the positive electrode; are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? learn the difference between anode and cathode, the electrodes of a cell or battery, and how to. Is The Red Electrode Positive Or Negative.
From www.makeuseof.com
Anode vs. Cathode Which Is Positive and Negative? Is The Red Electrode Positive Or Negative learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. in a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. the cathode is the positive electrode; are the electrodes of the (zn +2 /cu) cell that is described in. Is The Red Electrode Positive Or Negative.
From byjus.com
An electrolytic cell is used to convert Is The Red Electrode Positive Or Negative This seems reasonable as the anode. the cathode is the positive electrode; in a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. the pt electrode in the permanganate solution is the cathode; The anode is the negative electrode. During discharge, positive ions flow from anode to cathode. learn the difference. Is The Red Electrode Positive Or Negative.
From www.researchgate.net
Voltagecapacity (VQ) curves of the positive electrode and negative Is The Red Electrode Positive Or Negative This seems reasonable as the anode. During discharge, positive ions flow from anode to cathode. learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. The anode is the negative electrode. the cathode is the positive electrode; learn how to measure and interpret electrode and cell. Is The Red Electrode Positive Or Negative.
From www.dreamstime.com
Electrolysis Process Anode and Cathode Reactions Stock Illustration Is The Red Electrode Positive Or Negative The anode is the negative electrode. learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron. the cathode is the positive electrode; The one in the tin solution is the anode. This seems reasonable as the anode. the pt electrode in the permanganate solution. Is The Red Electrode Positive Or Negative.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students Is The Red Electrode Positive Or Negative During discharge, positive ions flow from anode to cathode. are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? the pt electrode in the permanganate solution is the cathode; in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the. Is The Red Electrode Positive Or Negative.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID9353258 Is The Red Electrode Positive Or Negative are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? During discharge, positive ions flow from anode to cathode. the cathode is the positive electrode; learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron.. Is The Red Electrode Positive Or Negative.
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? Is The Red Electrode Positive Or Negative The anode is the negative electrode. the pt electrode in the permanganate solution is the cathode; in a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? learn how to measure and. Is The Red Electrode Positive Or Negative.
From general.chemistrysteps.com
Electrolysis Chemistry Steps Is The Red Electrode Positive Or Negative in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently plate. learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. The one in the tin solution is the anode. the pt electrode in. Is The Red Electrode Positive Or Negative.
From www.thoughtco.com
How to Define Anode and Cathode Is The Red Electrode Positive Or Negative This seems reasonable as the anode. learn the difference between anode and cathode, the electrodes of a cell or battery, and how to remember which is which. the pt electrode in the permanganate solution is the cathode; are the electrodes of the (zn +2 /cu) cell that is described in figure 19.2.3 active or passive electrodes? . Is The Red Electrode Positive Or Negative.
From knovhov.com
How To Find Anode Cathode Of Diode 3 Testing Methods In Stepbystep Is The Red Electrode Positive Or Negative This seems reasonable as the anode. The one in the tin solution is the anode. in a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. The anode is the negative electrode. During discharge, positive ions flow from anode to cathode. are the electrodes of the (zn +2 /cu) cell that is described. Is The Red Electrode Positive Or Negative.
From slidetodoc.com
Define electrolysis The breakdown of an ionic compound Is The Red Electrode Positive Or Negative learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron. in a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. During discharge, positive ions flow from anode to cathode. the pt electrode in the permanganate solution is the. Is The Red Electrode Positive Or Negative.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an Is The Red Electrode Positive Or Negative the pt electrode in the permanganate solution is the cathode; in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently plate. During discharge, positive ions flow from anode to cathode. This seems reasonable as the anode. learn how to measure and interpret electrode and cell. Is The Red Electrode Positive Or Negative.