Emission Spectra Are Caused By . the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. In the early 20 th century, it was believed that the emission spectra of elements was caused by. this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Light from the sun sustains all forms of life on earth. It carries the visual information that makes. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure.
from stock.adobe.com
the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. Light from the sun sustains all forms of life on earth. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. In the early 20 th century, it was believed that the emission spectra of elements was caused by. this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. It carries the visual information that makes.
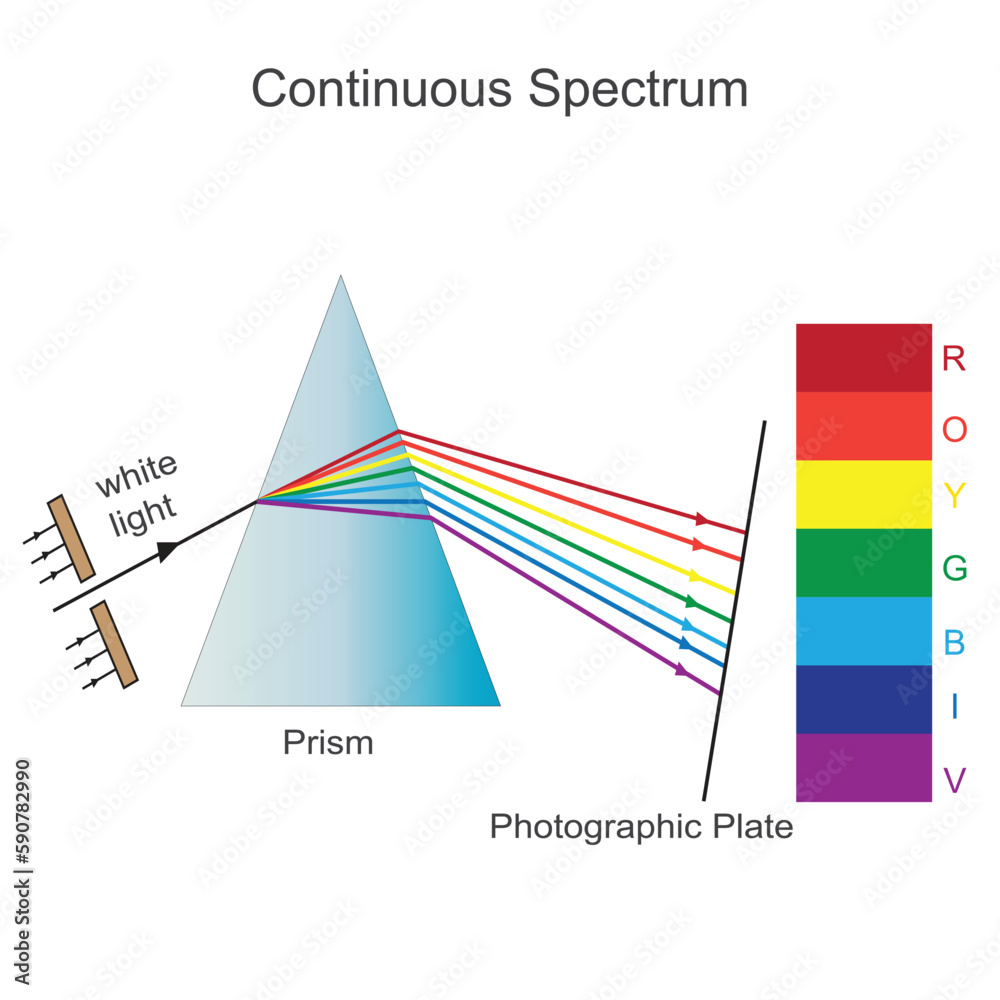
Vetor de Continuous spectrum, an emission spectrum that consists of
Emission Spectra Are Caused By Light from the sun sustains all forms of life on earth. It carries the visual information that makes. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. Light from the sun sustains all forms of life on earth. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. In the early 20 th century, it was believed that the emission spectra of elements was caused by.
From protonstalk.com
Difference Between Emission and Absorption Spectra ProtonsTalk Emission Spectra Are Caused By It carries the visual information that makes. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. In the early 20 th century, it was believed that the emission spectra of elements was caused by. emission and absorption spectra form the basis of spectroscopy, which uses spectra to. Emission Spectra Are Caused By.
From www.researchgate.net
Excitation/emission spectra of tracking dyes compatible with BDAA. (A Emission Spectra Are Caused By emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Light from the sun sustains all forms of life on earth. this is why. Emission Spectra Are Caused By.
From www.researchgate.net
Absorption and emission spectra of 13 in cyclohexane solution (top Emission Spectra Are Caused By Light from the sun sustains all forms of life on earth. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. It carries the visual information that makes. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected. Emission Spectra Are Caused By.
From www.mdpi.com
Molecules Free FullText Excited State of Benzo[a]pyrene Emission Spectra Are Caused By Light from the sun sustains all forms of life on earth. It carries the visual information that makes. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm. Emission Spectra Are Caused By.
From webbtelescope.org
Types of Spectra Continuous, Emission, and Absorption b Emission Spectra Are Caused By this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. the emission spectra are the energy spectra of the. Emission Spectra Are Caused By.
From laderarctic.weebly.com
Emission spectra laderarctic Emission Spectra Are Caused By In the early 20 th century, it was believed that the emission spectra of elements was caused by. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. It carries the visual information that makes. emission and absorption spectra form the basis of spectroscopy, which uses spectra to. Emission Spectra Are Caused By.
From www.researchgate.net
Emission spectra of the P1 solution (1 mg/mL) at different excitation Emission Spectra Are Caused By this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. It carries the visual information that makes. Light from the. Emission Spectra Are Caused By.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra Are Caused By Light from the sun sustains all forms of life on earth. In the early 20 th century, it was believed that the emission spectra of elements was caused by. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. emission and absorption spectra. Emission Spectra Are Caused By.
From www.researchgate.net
Emission spectra and DFT energies of the doublet (D1D3) states of the Emission Spectra Are Caused By Light from the sun sustains all forms of life on earth. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. this is why hydrogen’s emission spectrum is the. Emission Spectra Are Caused By.
From www.numerade.com
SOLVED Use the anthracene absorption and emission spectrum to Emission Spectra Are Caused By the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. It carries the visual information that makes. the emission spectrum (or line spectrum) of. Emission Spectra Are Caused By.
From www.researchgate.net
Emission spectra of pyrene and I/III ratio in different fatty esters Emission Spectra Are Caused By the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. It carries the visual information that makes. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. Light from the sun sustains all forms of. Emission Spectra Are Caused By.
From stock.adobe.com
Vetor de Continuous spectrum, an emission spectrum that consists of Emission Spectra Are Caused By this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. It carries the visual information that makes. the emission spectrum (or line spectrum) of. Emission Spectra Are Caused By.
From www.alamy.com
HHeHg emission spectra. Graphical representation of the emission Emission Spectra Are Caused By this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. In the early 20 th century, it was believed that the emission spectra of elements was caused by. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic. Emission Spectra Are Caused By.
From www.edinst.com
What are Absorption, Excitation and Emission Spectra? Emission Spectra Are Caused By It carries the visual information that makes. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. In the early 20 th century, it was believed that the emission spectra of elements was caused by. Light from the sun sustains all forms of life on earth. the emission. Emission Spectra Are Caused By.
From www.comsol.co.in
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Emission Spectra Are Caused By the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Light from the sun sustains all forms of life on earth. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. In the early 20. Emission Spectra Are Caused By.
From socratic.org
Question db4d1 Socratic Emission Spectra Are Caused By this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. Light from the sun sustains all forms of life on earth. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. It carries the visual information that. Emission Spectra Are Caused By.
From www.coursehero.com
[Solved] Figure 1 shows the emission spectra of five substances Emission Spectra Are Caused By In the early 20 th century, it was believed that the emission spectra of elements was caused by. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information. Emission Spectra Are Caused By.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle Emission Spectra Are Caused By this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. In the early 20 th century, it was believed that. Emission Spectra Are Caused By.
From www.pinterest.com
emission spectra periodic table Google Search AP Chem 5 Atomic Emission Spectra Are Caused By emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. Light from the sun sustains all forms of life on earth. In the early 20 th century, it was believed that the emission spectra of elements was caused by. the emission spectra are the energy spectra of the photons emitted. Emission Spectra Are Caused By.
From www.researchgate.net
Modelled emission spectra of atomic mercury at different electronic Emission Spectra Are Caused By this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. In the early 20 th century, it was believed that. Emission Spectra Are Caused By.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Are Caused By emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the. Emission Spectra Are Caused By.
From jeffery-has-robertson.blogspot.com
Describe the Cause of Atomic Emission Spectrum of an Element Jeffery Emission Spectra Are Caused By emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Light from the sun sustains all forms of life on earth. the emission spectra. Emission Spectra Are Caused By.
From www.esa.int
ESA Absorption and emission spectra of various elements Emission Spectra Are Caused By this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. It carries the visual information that makes. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. In the early. Emission Spectra Are Caused By.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Emission Spectra Are Caused By the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Light from the sun sustains all forms of life on earth. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. It carries the visual. Emission Spectra Are Caused By.
From www.researchgate.net
(a) Normalized emission, (b) actual emission, and (c) PLE spectra of Emission Spectra Are Caused By It carries the visual information that makes. Light from the sun sustains all forms of life on earth. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. the emission spectra are the energy spectra of the photons emitted during relaxation of the. Emission Spectra Are Caused By.
From www.hotzxgirl.com
Line Emission Spectrum How Is An Emission Spectrum Produced Study Hot Emission Spectra Are Caused By emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Light from the sun sustains all forms of life on earth. It carries the visual. Emission Spectra Are Caused By.
From www.reddit.com
Emission Spectra of the Elements in Periodic Table Format r/chemistry Emission Spectra Are Caused By emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. In the early 20 th century, it was believed that the emission spectra of elements was caused by. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected. Emission Spectra Are Caused By.
From www.researchgate.net
(a) The optimal excitation and emission spectra of the asprepared Emission Spectra Are Caused By this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. It carries the visual information that makes. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. the emission spectrum (or line spectrum) of. Emission Spectra Are Caused By.
From www.nagwa.com
Question Video Identifying a Spectrum as an Emission or Absorption Emission Spectra Are Caused By In the early 20 th century, it was believed that the emission spectra of elements was caused by. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. It carries. Emission Spectra Are Caused By.
From sciencephotogallery.com
Helium Emission And Absorption Spectra by Science Photo Library Emission Spectra Are Caused By Light from the sun sustains all forms of life on earth. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue),. It carries the visual information that. Emission Spectra Are Caused By.
From www.sciencephoto.com
Hydrogen emission and absorption spectra Stock Image C025/8082 Emission Spectra Are Caused By Light from the sun sustains all forms of life on earth. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. the emission spectra. Emission Spectra Are Caused By.
From myans.bhantedhammika.net
Emission Spectra For Hydrogen And Boron Atoms Emission Spectra Are Caused By emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. this is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm. Emission Spectra Are Caused By.
From www.nagwa.com
Lesson Video Emission and Absorption Spectra Nagwa Emission Spectra Are Caused By emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. In the early 20 th century, it was believed that the emission spectra of elements was caused by. this. Emission Spectra Are Caused By.
From rightmetal.weebly.com
Atomic emission spectrum chemistry definition rightmetal Emission Spectra Are Caused By In the early 20 th century, it was believed that the emission spectra of elements was caused by. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information. Emission Spectra Are Caused By.
From www.researchgate.net
Emission spectra of TC with various concentrations of Zn 2+ The Emission Spectra Are Caused By In the early 20 th century, it was believed that the emission spectra of elements was caused by. the emission spectra are the energy spectra of the photons emitted during relaxation of the excited electronic subsystem to the. Light from the sun sustains all forms of life on earth. emission and absorption spectra form the basis of spectroscopy,. Emission Spectra Are Caused By.