Titration Effect Definition . The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required. It provides valuable information about the. A method or the activity of finding exactly how much of a substance there is in a solution by…. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is.
from mirjamglessmer.com
A method or the activity of finding exactly how much of a substance there is in a solution by…. The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. It provides valuable information about the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown.
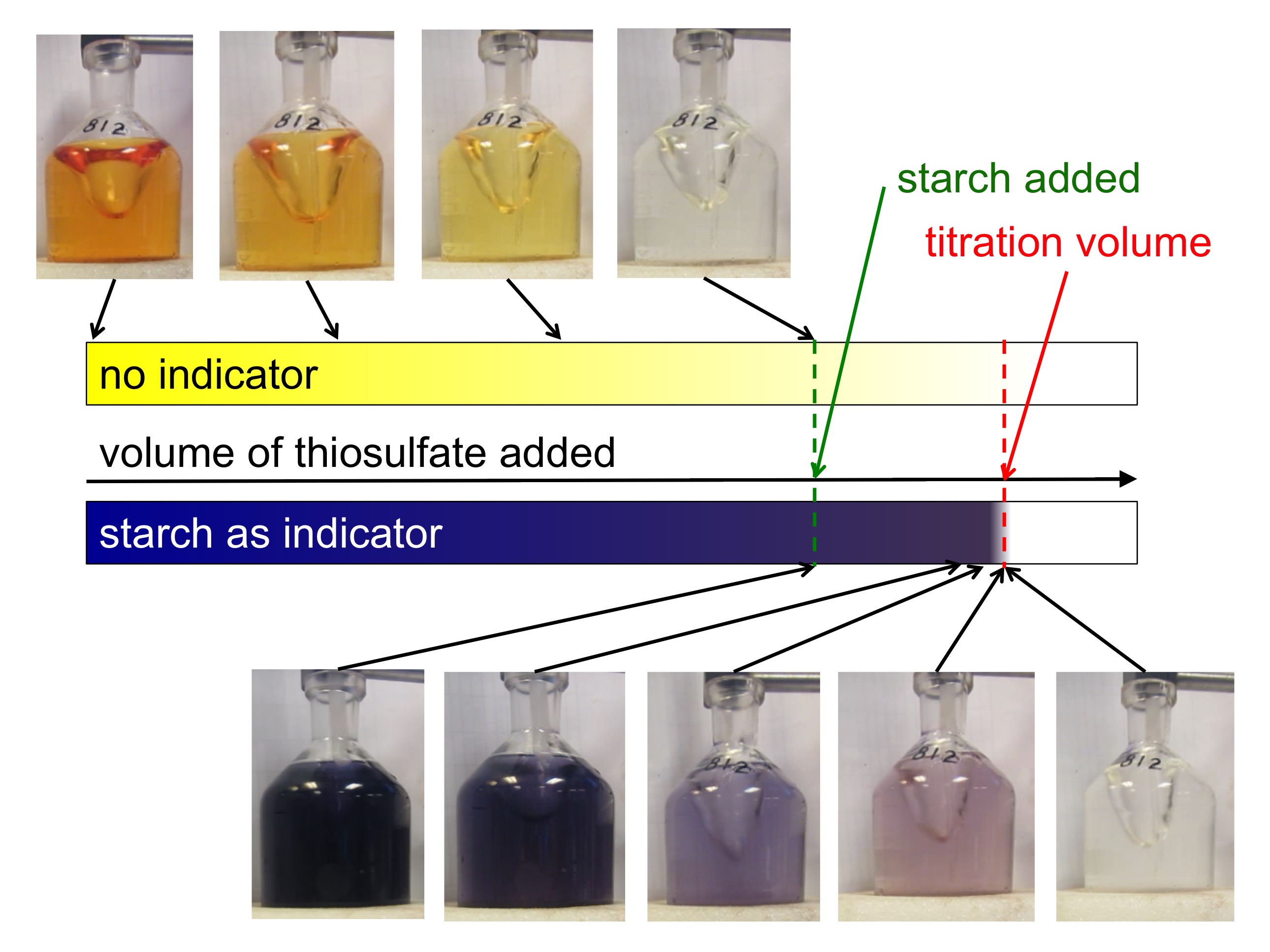
Measuring the concentration of dissolved oxygen in sea water Part 3
Titration Effect Definition It provides valuable information about the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required. A method or the activity of finding exactly how much of a substance there is in a solution by…. It provides valuable information about the.
From dxolbdanx.blob.core.windows.net
What Is A Titration For Dummies at Jimmie Cook blog Titration Effect Definition The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. It provides valuable information about the. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A method or the activity of finding exactly how much of a. Titration Effect Definition.
From chemistnotes.com
Complexometric Titration Definition, Types, Indicators, and 5 Reliable Titration Effect Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. It provides valuable information about the. Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. Titration Effect Definition.
From www.youtube.com
Total Water Hardness using EDTA Titration YouTube Titration Effect Definition It provides valuable information about the. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. A method or the activity of finding exactly how much of a substance there. Titration Effect Definition.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Titration Effect Definition It provides valuable information about the. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution. Titration Effect Definition.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Effect Definition It provides valuable information about the. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of. Titration Effect Definition.
From www.tes.com
KS3 GCSE Chemistry Introduction To Titrations Lesson Presentation and Titration Effect Definition Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required. The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. A method or the activity of finding exactly. Titration Effect Definition.
From chem.libretexts.org
3.7 AcidBase Titrations Chemistry LibreTexts Titration Effect Definition The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The titration curve is a graphical representation of the ph or other. Titration Effect Definition.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Effect Definition A method or the activity of finding exactly how much of a substance there is in a solution by…. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. A. Titration Effect Definition.
From studylib.net
Titration Curve weak base with strong acid START Titration Effect Definition Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. It provides valuable information about the. Titration is the slow addition of one solution. Titration Effect Definition.
From www.thoughtco.com
Titration Definition (Chemistry) Titration Effect Definition A method or the activity of finding exactly how much of a substance there is in a solution by…. The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. The titration curve is a graphical representation of the ph or other property changes during a titration. Titration Effect Definition.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog Titration Effect Definition It provides valuable information about the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A method or the activity of finding exactly how much of a substance there is in a solution by…. The titration curve is. Titration Effect Definition.
From bazaemmalyman.blogspot.com
Back Titration Method Emma Lyman Titration Effect Definition Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required. The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. Titration is the slow addition of one solution. Titration Effect Definition.
From moodle.tau.ac.il
AcidBase Titration Curves Titration Effect Definition It provides valuable information about the. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. Titration is defined as a method or process of determining the concentration. Titration Effect Definition.
From www.slideserve.com
PPT Ch 6 Good Titrations PowerPoint Presentation, free download ID Titration Effect Definition The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. Titration is. Titration Effect Definition.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Effect Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required. It provides valuable information about the. Titration, process. Titration Effect Definition.
From chemistnotes.com
Redox Titration Definition, Requirements, Indicators, and 5 Reliable Titration Effect Definition It provides valuable information about the. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. Titration is defined as a method or process of determining the concentration. Titration Effect Definition.
From exoluaxpt.blob.core.windows.net
Titration Kid Definition Chemistry at Linda Arguello blog Titration Effect Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required. Titration,. Titration Effect Definition.
From mirjamglessmer.com
Measuring the concentration of dissolved oxygen in sea water Part 3 Titration Effect Definition It provides valuable information about the. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. A method or the activity of finding exactly how much of a substance there is in a solution by…. The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms. Titration Effect Definition.
From labthink.netlify.app
What is titration and how does it work Titration Effect Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. It provides valuable information about the. A method or the activity of finding exactly how much of a substance there is in a solution by…. Titration, process of chemical analysis in which the quantity of some. Titration Effect Definition.
From www.youtube.com
Titration Definition, steps and formula YouTube Titration Effect Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is. Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required. A method or the activity of finding exactly how much of a substance there is in. Titration Effect Definition.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts Titration Effect Definition The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is defined as a method or process of determining the concentration. Titration Effect Definition.
From www.youtube.com
Titrations Analytical Techniques Me Study YouTube Titration Effect Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. It provides valuable information about. Titration Effect Definition.
From celtjcvb.blob.core.windows.net
Titration In Simple Terms at John Haslett blog Titration Effect Definition The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A method or the activity of finding exactly how much of a substance there is in a solution by…. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. A titration is a volumetric technique in. Titration Effect Definition.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Titration Effect Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A method or the activity. Titration Effect Definition.
From joizlgxlm.blob.core.windows.net
Titration Experiments In Supramolecular Chemistry at Larry Ferrill blog Titration Effect Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the. Titration Effect Definition.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Effect Definition A method or the activity of finding exactly how much of a substance there is in a solution by…. Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required. It provides valuable information about the. Titration is the slow addition of one. Titration Effect Definition.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Titration Effect Definition A method or the activity of finding exactly how much of a substance there is in a solution by…. It provides valuable information about the. Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required. Titration is the slow addition of one. Titration Effect Definition.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Effect Definition It provides valuable information about the. The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required. Titration, process. Titration Effect Definition.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Effect Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is defined as a. Titration Effect Definition.
From www.youtube.com
Chemistry & Biology Define the Term Titration YouTube Titration Effect Definition The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A method or the activity of finding exactly how much of a substance there is in a solution by…. Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. Titration Effect Definition.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Effect Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A method or the activity. Titration Effect Definition.
From relationshipbetween.com
Difference Between Titration And Neutralization Relationship Between Titration Effect Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is. Titration is defined as a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of reagent of known concentration required. A method or the activity of finding exactly how much of a substance there is in. Titration Effect Definition.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Titration Effect Definition The meaning of titration is a method or process of determining the concentration of a dissolved substance in terms of the smallest amount of. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. It provides. Titration Effect Definition.
From chemistnotes.com
Complexometric Titration Definition, Types, Indicators, and 5 Reliable Titration Effect Definition The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Titration Effect Definition.
From www.numerade.com
SOLVED Define the following terms used in volumetric/titrimetric Titration Effect Definition It provides valuable information about the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. The titration curve is a graphical representation of the ph or other property changes. Titration Effect Definition.