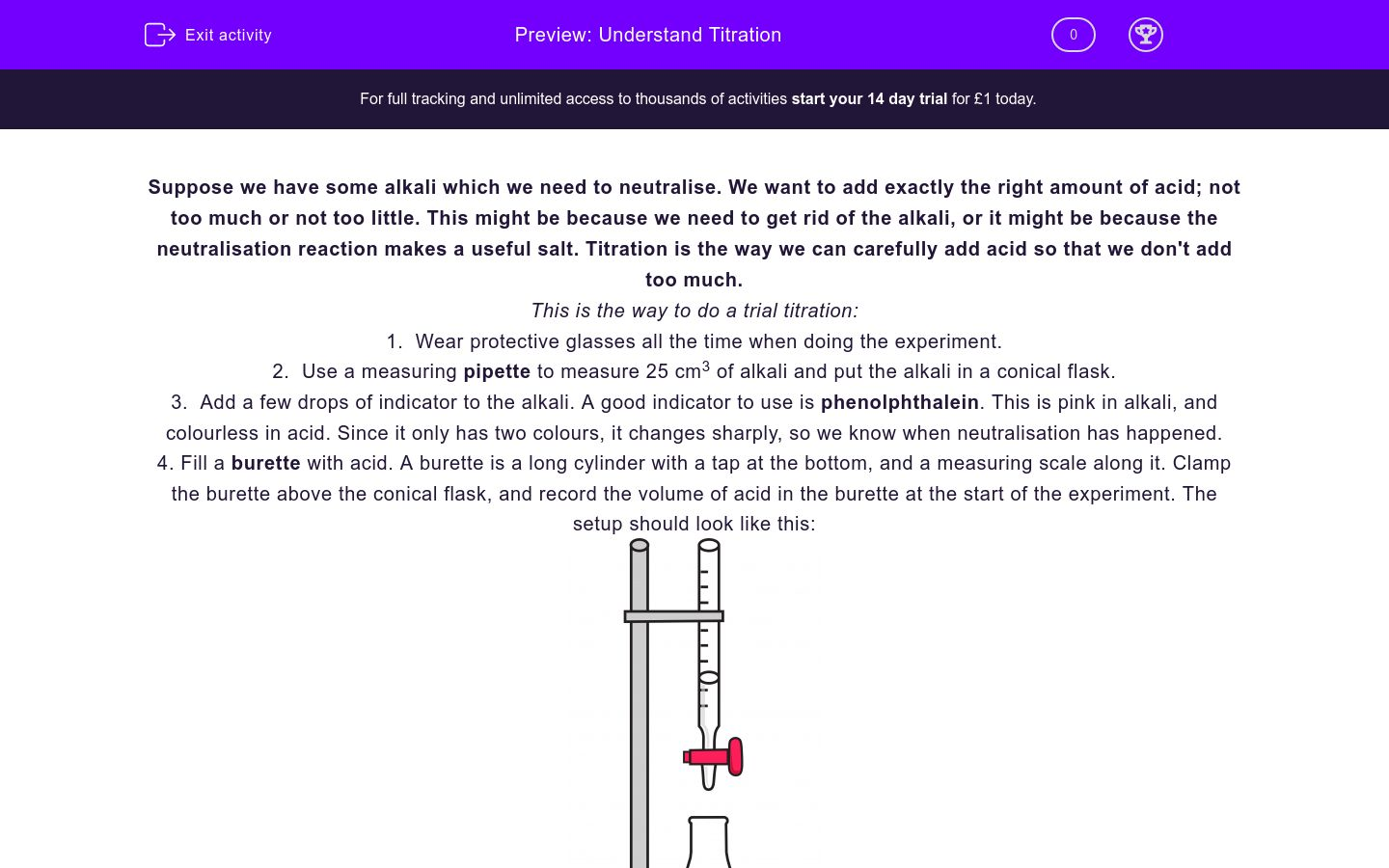
Titration Questions Worksheet . titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely measured volume of a solution with unknown concentration (the analyte) to react. consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. titrations are a very accurate way of measuring the concentration of acids and alkalis. In a titration, we measure the. If it takes 54 ml of 0.1 m naoh. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. (a) write the equation for the. Find the requested quantities in the following problems:
from www.edplace.com
consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. In a titration, we measure the. Find the requested quantities in the following problems: titrations are a very accurate way of measuring the concentration of acids and alkalis. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely measured volume of a solution with unknown concentration (the analyte) to react. (a) write the equation for the. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. If it takes 54 ml of 0.1 m naoh.
Understand Titration Worksheet EdPlace
Titration Questions Worksheet titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. titrations are a very accurate way of measuring the concentration of acids and alkalis. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely measured volume of a solution with unknown concentration (the analyte) to react. (a) write the equation for the. consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. If it takes 54 ml of 0.1 m naoh. In a titration, we measure the. Find the requested quantities in the following problems: 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution.
From www.edplace.com
Understand Titration Worksheet EdPlace Titration Questions Worksheet In a titration, we measure the. consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. If it takes 54 ml of 0.1 m naoh. titrations are a very accurate way of measuring the concentration. Titration Questions Worksheet.
From www.chegg.com
Solved EXPERIMENT 10 WORKSHEET It's Back!... Titrations Titration Questions Worksheet titrations are a very accurate way of measuring the concentration of acids and alkalis. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. In a titration, we measure the. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely measured volume of. Titration Questions Worksheet.
From www.studypool.com
SOLUTION Titration Student Exploration Worksheet Studypool Titration Questions Worksheet titrations are a very accurate way of measuring the concentration of acids and alkalis. In a titration, we measure the. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. consider the titration. Titration Questions Worksheet.
From www.studypool.com
SOLUTION Chemsheets gcse 1105 titrations 1 ans 93ghs Studypool Titration Questions Worksheet titrations are a very accurate way of measuring the concentration of acids and alkalis. consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely measured volume of a solution with unknown concentration (the analyte) to. Titration Questions Worksheet.
From www.housview.com
Titration Practice Worksheet Worksheets For All Free Worksheets Samples Titration Questions Worksheet A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. If it takes 54 ml of 0.1 m naoh. consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. titrations are a very accurate way of measuring the concentration of acids and alkalis. Find the requested quantities. Titration Questions Worksheet.
From rocketsheets.co.uk
Titrations Home Learning Worksheet GCSE rocketsheets.co.uk Titration Questions Worksheet 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. A 1.0000 gram sample of k2co3 (138.2055. Titration Questions Worksheet.
From studylib.net
Titration Worksheet Titration Questions Worksheet Find the requested quantities in the following problems: In a titration, we measure the. If it takes 54 ml of 0.1 m naoh. titrations are a very accurate way of measuring the concentration of acids and alkalis. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. titration is the addition of. Titration Questions Worksheet.
From www.chemistrylearner.com
Free Printable Acids and Bases Titration Worksheets Titration Questions Worksheet If it takes 54 ml of 0.1 m naoh. (a) write the equation for the. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. titrations are a very accurate way of measuring the concentration. Titration Questions Worksheet.
From education-com-worksheets.blogspot.com
Acid Base Titration Worksheet Answers Education Com Worksheets Titration Questions Worksheet If it takes 54 ml of 0.1 m naoh. Find the requested quantities in the following problems: In a titration, we measure the. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. titration is the addition. Titration Questions Worksheet.
From www.tes.com
Titrations Home Learning Worksheet GCSE Teaching Resources Titration Questions Worksheet consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. (a) write the equation for the. If it takes 54 ml of 0.1 m naoh. In a titration, we measure the. 1) it takes 83 ml. Titration Questions Worksheet.
From www.chegg.com
Solved EXPERIMENT 10 WORKSHEET It's Back!... Titrations Titration Questions Worksheet titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. In a titration, we measure the. titrations are a very accurate way of measuring the concentration of acids and alkalis. consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. titration is the addition. Titration Questions Worksheet.
From www.scribd.com
gizmo titration worksheet Titration Ph Titration Questions Worksheet A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. In a titration, we measure the. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely measured volume of a solution with unknown concentration (the analyte) to react. titration is the addition of a standard. Titration Questions Worksheet.
From studylib.net
acid base titration worksheet answer key Titration Questions Worksheet If it takes 54 ml of 0.1 m naoh. titrations are a very accurate way of measuring the concentration of acids and alkalis. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. Titration Questions Worksheet.
From www.chegg.com
Solved Titration Of Strong And Weak Acids Smart Worksheet Titration Questions Worksheet titrations are a very accurate way of measuring the concentration of acids and alkalis. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely measured volume of a solution with unknown concentration (the analyte) to. Titration Questions Worksheet.
From worksheetcampusslewed.z21.web.core.windows.net
Chemistry Titration Questions And Answers Titration Questions Worksheet consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. If it takes 54 ml of 0.1 m naoh. Find the requested quantities in the following problems: titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. titrations are a very accurate way of measuring. Titration Questions Worksheet.
From www.chegg.com
Solved Titration Curves Worksheet The following are Weak Titration Questions Worksheet If it takes 54 ml of 0.1 m naoh. In a titration, we measure the. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. (a) write the equation for the. Find the requested quantities. Titration Questions Worksheet.
From docworksheet.com
Gcse Titration Calculations Worksheet Titration Questions Worksheet (a) write the equation for the. If it takes 54 ml of 0.1 m naoh. In a titration, we measure the. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely measured volume of. Titration Questions Worksheet.
From studylib.net
Titration Practice Worksheet Titration Questions Worksheet A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. titrations are a very accurate way. Titration Questions Worksheet.
From edzion.com
HSC Titration Questions Edzion Titration Questions Worksheet A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. titrations are a very accurate way of measuring the concentration of acids and alkalis. Find the requested quantities in the following problems: titration is the addition. Titration Questions Worksheet.
From worksheets.clipart-library.com
Acid base titration worksheet Live Worksheets Worksheets Library Titration Questions Worksheet 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely measured volume of a solution with unknown concentration (the analyte) to react. titrations are a very accurate way of measuring the concentration. Titration Questions Worksheet.
From worksheetcampusslewed.z21.web.core.windows.net
Chemistry Titration Questions And Answers Titration Questions Worksheet Find the requested quantities in the following problems: If it takes 54 ml of 0.1 m naoh. In a titration, we measure the. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. (a) write the equation for the. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water. Titration Questions Worksheet.
From www.scribd.com
Redox Titration Quiz PDF Titration Chemistry Titration Questions Worksheet Find the requested quantities in the following problems: If it takes 54 ml of 0.1 m naoh. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. (a) write the equation for the. titrations are a very accurate way of measuring the concentration of acids and alkalis. In a titration,. Titration Questions Worksheet.
From www.vrogue.co
Titration Calculations And Questions Worksheet Teachi vrogue.co Titration Questions Worksheet Find the requested quantities in the following problems: (a) write the equation for the. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely measured volume of a solution with unknown concentration (the analyte) to react. If it takes 54 ml of 0.1 m naoh. consider the titration of 25.00 ml. Titration Questions Worksheet.
From www.chemistrylearner.com
Free Printable Acids and Bases Titration Worksheets Titration Questions Worksheet titrations are a very accurate way of measuring the concentration of acids and alkalis. (a) write the equation for the. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Find the requested quantities in the following problems: If it takes 54 ml of 0.1 m naoh. titration is. Titration Questions Worksheet.
From www.chemistryworksheet.com
Chemistry Titration Worksheet Answers Titration Questions Worksheet In a titration, we measure the. Find the requested quantities in the following problems: consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. titration is the addition of a standard solution of precisely. Titration Questions Worksheet.
From studylib.net
Titration worksheet Titration Questions Worksheet titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. In a titration, we measure the. Find the requested quantities in the following problems: A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. titrations are a very accurate way of measuring the concentration of. Titration Questions Worksheet.
From www.vrogue.co
Titration Practice Worksheet Worksheets For All Free vrogue.co Titration Questions Worksheet titrations are a very accurate way of measuring the concentration of acids and alkalis. If it takes 54 ml of 0.1 m naoh. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. titration is the addition of a standard solution of precisely known concentration (the titrant) to a. Titration Questions Worksheet.
From www.tes.com
Titrations Home Learning Worksheet GCSE Teaching Resources Titration Questions Worksheet 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely measured volume of a solution with unknown concentration (the analyte) to react. Find the requested quantities in the following problems: consider the. Titration Questions Worksheet.
From wks.udlvirtual.edu.pe
Acid Base Titration Practice Worksheet Worksheets Printable Free Titration Questions Worksheet titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Find the requested quantities in the following problems: If it takes 54 ml of 0.1 m naoh. titrations are a very accurate. Titration Questions Worksheet.
From studylib.net
Titrations Practice Worksheet Titration Questions Worksheet Find the requested quantities in the following problems: In a titration, we measure the. titrations are a very accurate way of measuring the concentration of acids and alkalis. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. titration is the addition of a standard solution of precisely known concentration. Titration Questions Worksheet.
From www.chegg.com
AcidBase Titration Worksheet following titrations. Titration Questions Worksheet If it takes 54 ml of 0.1 m naoh. (a) write the equation for the. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely measured volume of a solution with unknown concentration (the analyte) to react. Find the requested quantities in the following problems: titration is the addition of a. Titration Questions Worksheet.
From www.studypool.com
SOLUTION Igcse chem topic questions titration Studypool Titration Questions Worksheet consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. Find the requested quantities in the following problems: A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. If it takes 54 ml of 0.1 m naoh. In a titration, we measure the. (a) write the equation for. Titration Questions Worksheet.
From philo-khosravani.blogspot.com
Acid Base Titration Worksheet Answers philokhosravani Titration Questions Worksheet In a titration, we measure the. titrations are a very accurate way of measuring the concentration of acids and alkalis. If it takes 54 ml of 0.1 m naoh. Find the requested quantities in the following problems: (a) write the equation for the. titration is the addition of a standard solution of precisely known concentration (the titrant) to. Titration Questions Worksheet.
From blogs.glowscotland.org.uk
Acid & Alkali Revision (incl. Titration Calculation) National 5 Chemistry Skills & Revision Titration Questions Worksheet titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. If it takes 54 ml of 0.1 m naoh. titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely measured volume of a solution with unknown concentration (the analyte) to react. (a) write the. Titration Questions Worksheet.
From study.com
Quiz & Worksheet Overview of Titration Titration Questions Worksheet titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. consider the titration of 25.00 ml of 0.1000 m acetic acid with 0.1000 m naoh. (a) write the equation for the. Find the requested quantities in the following problems: titration is the addition of a standard solution of precisely known. Titration Questions Worksheet.