Medical Device Classification Definitions . Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The united states food and drug administration (fda) categorizes medical devices into three classes: The food and drug administration (fda) has established classifications for approximately 1,700 different. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. The fda classifies medical devices. As simple as a tongue depressor. Fda, the european commission, and health canada. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. Class i, class ii, or class iii.
from www.arenasolutions.com
The food and drug administration (fda) has established classifications for approximately 1,700 different. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. The fda classifies medical devices. Class i, class ii, or class iii. Fda, the european commission, and health canada. The united states food and drug administration (fda) categorizes medical devices into three classes: As simple as a tongue depressor.
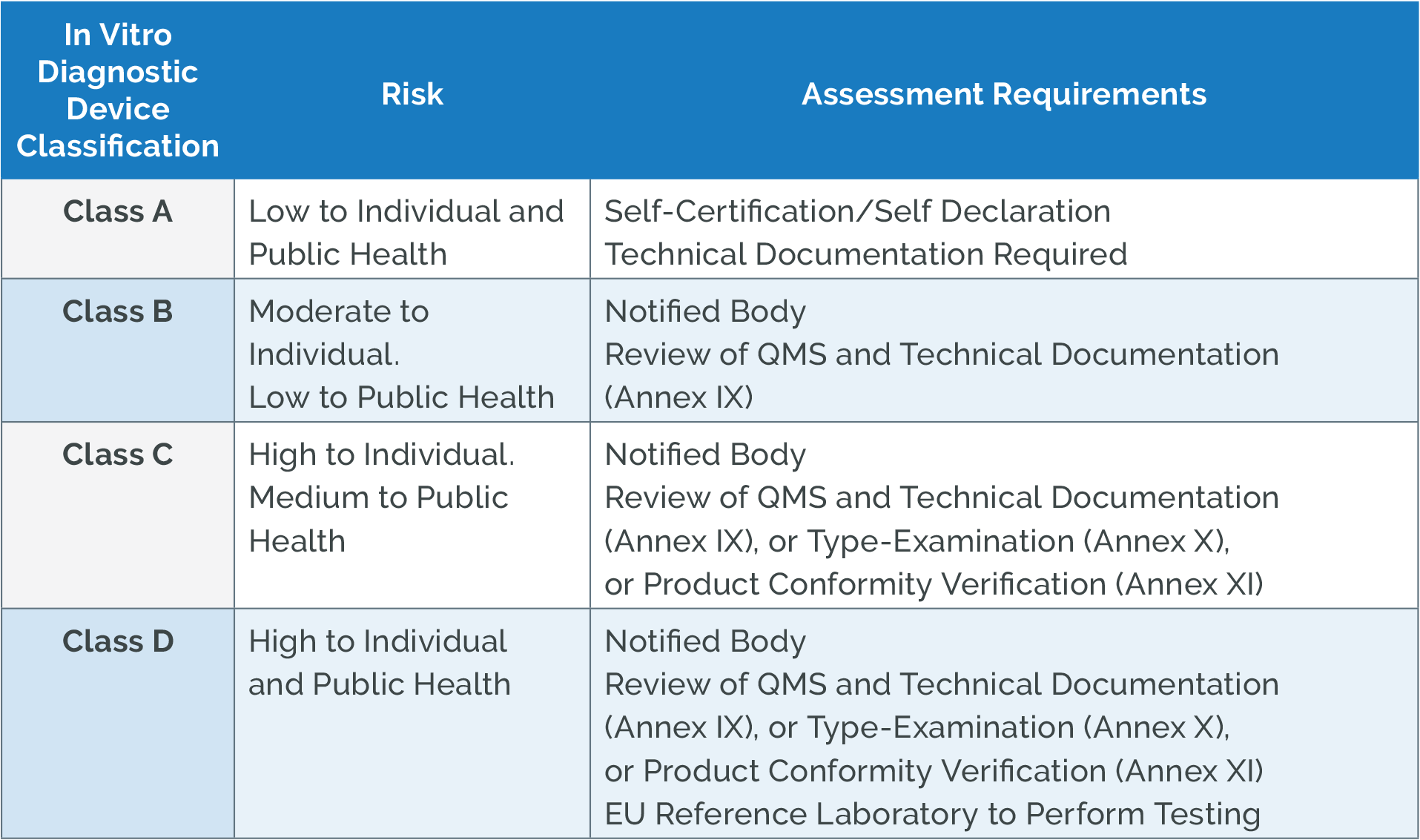
How to Classify Your Medical Device Under the EU MDR and IVDR Arena
Medical Device Classification Definitions Class i, class ii, or class iii. The united states food and drug administration (fda) categorizes medical devices into three classes: Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. As simple as a tongue depressor. The food and drug administration (fda) has established classifications for approximately 1,700 different. Class i, class ii, or class iii. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The fda classifies medical devices. Fda, the european commission, and health canada.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment Medical Device Classification Definitions As simple as a tongue depressor. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. Class i, class ii, or class iii. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The fda. Medical Device Classification Definitions.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Medical Device Classification Definitions The fda classifies medical devices. Class i, class ii, or class iii. As simple as a tongue depressor. The food and drug administration (fda) has established classifications for approximately 1,700 different. The united states food and drug administration (fda) categorizes medical devices into three classes: Fda, the european commission, and health canada. Such a device will be classified by regulation. Medical Device Classification Definitions.
From mungfali.com
Classification Of Medical Devices Medical Device Classification Definitions The united states food and drug administration (fda) categorizes medical devices into three classes: Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. The. Medical Device Classification Definitions.
From rs-ness.com
IVD Classification Under IVDR Definition RS NESS Medical Device Classification Definitions The united states food and drug administration (fda) categorizes medical devices into three classes: Fda, the european commission, and health canada. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. The food and drug administration (fda) has established classifications for approximately 1,700 different. Classification of a. Medical Device Classification Definitions.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Medical Device Classification Definitions The united states food and drug administration (fda) categorizes medical devices into three classes: Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization.. Medical Device Classification Definitions.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Classification Definitions Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. As simple as a tongue depressor. The fda classifies medical devices. The united states. Medical Device Classification Definitions.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Classification Definitions Fda, the european commission, and health canada. The food and drug administration (fda) has established classifications for approximately 1,700 different. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. The united states food and drug administration (fda) categorizes medical devices into three classes: Classification of a. Medical Device Classification Definitions.
From www.researchgate.net
Classification of medical device technology users Download Scientific Medical Device Classification Definitions Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. Fda, the european commission, and health canada. The fda classifies medical devices. As simple as. Medical Device Classification Definitions.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Classification Definitions As simple as a tongue depressor. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The fda classifies medical devices. Class i, class ii, or class iii. The united states food and drug administration (fda) categorizes medical devices into three classes: The food and drug administration (fda). Medical Device Classification Definitions.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Medical Device Classification Definitions Fda, the european commission, and health canada. The fda classifies medical devices. As simple as a tongue depressor. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or. Medical Device Classification Definitions.
From www.researchgate.net
Medical Device Classification a Download Scientific Diagram Medical Device Classification Definitions As simple as a tongue depressor. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. The fda classifies medical devices. The food and drug administration (fda) has established classifications for approximately 1,700 different. Such a device will be classified by regulation into either class i (general. Medical Device Classification Definitions.
From www.presentationeze.com
TGA Medical Device Classification Rule 5 Special RulesPresentationEZE Medical Device Classification Definitions As simple as a tongue depressor. Fda, the european commission, and health canada. The fda classifies medical devices. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by. Medical Device Classification Definitions.
From english.nmpa.gov.cn
Rules for Classification of Medical Devices Medical Device Classification Definitions Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. Fda, the european commission, and health canada. Class i, class ii, or class iii. The fda classifies medical devices. As simple as a tongue depressor. Classification of a medical device (21 cfr 860) medical devices are regulated. Medical Device Classification Definitions.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Medical Device Classification Definitions The fda classifies medical devices. Class i, class ii, or class iii. As simple as a tongue depressor. The united states food and drug administration (fda) categorizes medical devices into three classes: Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. Fda, the european commission, and. Medical Device Classification Definitions.
From mavink.com
Medical Device Classification Chart Medical Device Classification Definitions The food and drug administration (fda) has established classifications for approximately 1,700 different. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. Class i, class ii, or class iii. As simple as a tongue depressor. Fda, the european commission, and health canada. The united states food. Medical Device Classification Definitions.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Medical Device Classification Definitions Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. Fda, the european commission, and health canada. The fda classifies medical devices. As simple as a tongue depressor. The united states food and drug administration (fda) categorizes medical devices into three classes: Such a device will be. Medical Device Classification Definitions.
From www.avanti-europe.ch
What you need to know about the MDR classification rules Medical Device Classification Definitions The fda classifies medical devices. Class i, class ii, or class iii. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. As simple as a tongue depressor. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class. Medical Device Classification Definitions.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Classification Definitions Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The food and drug administration (fda) has established classifications for approximately 1,700 different. The fda classifies medical devices. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical. Medical Device Classification Definitions.
From www.researchgate.net
5.3 Overview on the classification of medical devices and the Medical Device Classification Definitions Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. The fda classifies medical devices. Fda, the european commission, and health canada. As simple as a tongue depressor. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by. Medical Device Classification Definitions.
From mavink.com
Mdr Classification Chart Medical Device Classification Definitions Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. The fda classifies medical devices. Class i, class ii, or class iii. Fda, the. Medical Device Classification Definitions.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Device Classification Definitions As simple as a tongue depressor. The fda classifies medical devices. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. The food and drug administration (fda) has established classifications for approximately 1,700 different. Fda, the european commission, and health canada. Class i, class ii, or class. Medical Device Classification Definitions.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Classification Definitions Fda, the european commission, and health canada. The food and drug administration (fda) has established classifications for approximately 1,700 different. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes. Medical Device Classification Definitions.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Classification Definitions Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The fda classifies medical devices. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. The united states food and drug administration (fda) categorizes medical. Medical Device Classification Definitions.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Medical Device Classification Definitions As simple as a tongue depressor. Class i, class ii, or class iii. The fda classifies medical devices. Fda, the european commission, and health canada. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Such a device will be classified by regulation into either class i (general. Medical Device Classification Definitions.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Medical Device Classification Definitions The fda classifies medical devices. As simple as a tongue depressor. Class i, class ii, or class iii. The united states food and drug administration (fda) categorizes medical devices into three classes: Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Fda, the european commission, and health. Medical Device Classification Definitions.
From es.slideshare.net
Regulation of Medical Devices in US Medical Device Classification Definitions Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. The food and drug administration (fda) has established classifications for approximately 1,700 different. As simple as a tongue depressor. Fda, the european commission, and health canada. The united states food and drug administration (fda) categorizes medical devices. Medical Device Classification Definitions.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Device Classification Definitions Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The fda classifies medical devices. As simple as a tongue depressor. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. The food and drug. Medical Device Classification Definitions.
From laegemiddelstyrelsen.dk
Medical devices Medical Device Classification Definitions Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. Fda, the european commission, and health canada. The food and drug administration (fda) has established classifications for approximately 1,700 different. Class i, class ii, or class iii. The united states food and drug administration (fda) categorizes medical. Medical Device Classification Definitions.
From mavink.com
Fda Medical Device Classification Chart Medical Device Classification Definitions Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The fda classifies medical devices. Fda, the european commission, and health canada. The united states. Medical Device Classification Definitions.
From www.arenasolutions.com
How to Classify Your Medical Device Under the EU MDR and IVDR Arena Medical Device Classification Definitions The food and drug administration (fda) has established classifications for approximately 1,700 different. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. The united states food and drug administration (fda) categorizes medical devices into three classes: Such a device will be classified by regulation into either. Medical Device Classification Definitions.
From medicaldevicehq.com
Different classifications rules for medical device software An Medical Device Classification Definitions The united states food and drug administration (fda) categorizes medical devices into three classes: The fda classifies medical devices. The food and drug administration (fda) has established classifications for approximately 1,700 different. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Section 201(h) of the fdca defines. Medical Device Classification Definitions.
From mungfali.com
Classification Of Medical Devices Medical Device Classification Definitions Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. As simple as a tongue depressor. Fda, the european commission, and health canada. The food and drug administration (fda) has established classifications for approximately 1,700 different. The fda classifies medical devices. Such a device will be classified by. Medical Device Classification Definitions.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Medical Device Classification Definitions Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. Fda, the european commission, and health canada. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. The fda classifies medical devices. The united. Medical Device Classification Definitions.
From spyro-soft.com
EU MDR everything you need to know about Medical Device Regulation Medical Device Classification Definitions The united states food and drug administration (fda) categorizes medical devices into three classes: As simple as a tongue depressor. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or metabolization. The fda classifies medical devices. Such a device will be classified by regulation into either class i. Medical Device Classification Definitions.
From www.pacificbridgemedical.com
Device Classification in India Infographic Medical Device Classification Definitions The food and drug administration (fda) has established classifications for approximately 1,700 different. The united states food and drug administration (fda) categorizes medical devices into three classes: Fda, the european commission, and health canada. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Section 201(h) of. Medical Device Classification Definitions.