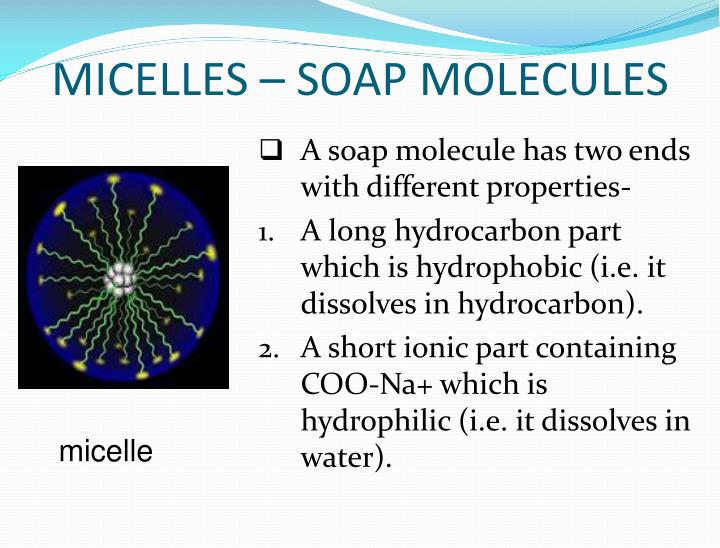
Soaps Are Long Chain Of . Cleansing action of soaps and detergents. Previously, the alkali which was needed to. The most common examples of such compounds are soaps and detergents, four of which are shown below. Soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The molecule of soap constitutes. Soaps act as cleansers because the two ends of a soap molecule are so different. Most of the dirt is oily in nature and oil does not dissolve in water. Generally, soap is created from a long chain of carbon atoms that carry two hydrogen atoms. Each soap molecule has a long. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). Updated on july 19, 2019. They are surfactants (compounds that reduce the surface tension between a. The reaction is called saponification and produces. Note that each of these molecules has a nonpolar hydrocarbon chain,.
from www.slideserve.com
Updated on july 19, 2019. Cleansing action of soaps and detergents. They are surfactants (compounds that reduce the surface tension between a. The most common examples of such compounds are soaps and detergents, four of which are shown below. Previously, the alkali which was needed to. The molecule of soap constitutes. Each soap molecule has a long. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). Soaps act as cleansers because the two ends of a soap molecule are so different. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification.
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID3090261
Soaps Are Long Chain Of Soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. Most of the dirt is oily in nature and oil does not dissolve in water. The reaction is called saponification and produces. Previously, the alkali which was needed to. They are surfactants (compounds that reduce the surface tension between a. Note that each of these molecules has a nonpolar hydrocarbon chain,. Soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. The molecule of soap constitutes. Generally, soap is created from a long chain of carbon atoms that carry two hydrogen atoms. Soaps act as cleansers because the two ends of a soap molecule are so different. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). The most common examples of such compounds are soaps and detergents, four of which are shown below. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Each soap molecule has a long. Cleansing action of soaps and detergents. Updated on july 19, 2019.
From www.scribd.com
Experiment 13 Preparation of Soap Soaps Are Carboxylate Salts With Soaps Are Long Chain Of Updated on july 19, 2019. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). Cleansing action of soaps and detergents. Soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. They are surfactants (compounds that reduce the surface tension between a. The reaction is called saponification and. Soaps Are Long Chain Of.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Soaps Are Long Chain Of The most common examples of such compounds are soaps and detergents, four of which are shown below. Most of the dirt is oily in nature and oil does not dissolve in water. The molecule of soap constitutes. Soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. Updated on july 19, 2019.. Soaps Are Long Chain Of.
From www.slideshare.net
How do soaps work Soaps Are Long Chain Of Most of the dirt is oily in nature and oil does not dissolve in water. Cleansing action of soaps and detergents. The most common examples of such compounds are soaps and detergents, four of which are shown below. They are surfactants (compounds that reduce the surface tension between a. Each soap molecule has a long. Soap is made via a. Soaps Are Long Chain Of.
From www.teachoo.com
[Class 10] Soaps and Detergents Structure, Cleansing Action and more Soaps Are Long Chain Of Generally, soap is created from a long chain of carbon atoms that carry two hydrogen atoms. Each soap molecule has a long. The molecule of soap constitutes. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Note that each of these molecules has a nonpolar hydrocarbon chain,. Previously, the. Soaps Are Long Chain Of.
From www.kenarry.com
Handmade Soap Chains An Easy DIY Gift Idea and Tutorial Soaps Are Long Chain Of Updated on july 19, 2019. The reaction is called saponification and produces. Each soap molecule has a long. Cleansing action of soaps and detergents. Generally, soap is created from a long chain of carbon atoms that carry two hydrogen atoms. Soaps act as cleansers because the two ends of a soap molecule are so different. Soap is made via a. Soaps Are Long Chain Of.
From www.researchgate.net
Flow diagram of soap manufacturing process Download Scientific Diagram Soaps Are Long Chain Of Each soap molecule has a long. Previously, the alkali which was needed to. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of these molecules has a nonpolar hydrocarbon chain,. Updated on july 19, 2019. Generally, soap is created from a long chain of carbon atoms that carry two. Soaps Are Long Chain Of.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents Soaps Are Long Chain Of Cleansing action of soaps and detergents. The reaction is called saponification and produces. Most of the dirt is oily in nature and oil does not dissolve in water. They are surfactants (compounds that reduce the surface tension between a. Note that each of these molecules has a nonpolar hydrocarbon chain,. Previously, the alkali which was needed to. Generally, soap is. Soaps Are Long Chain Of.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID Soaps Are Long Chain Of The molecule of soap constitutes. The most common examples of such compounds are soaps and detergents, four of which are shown below. Soaps act as cleansers because the two ends of a soap molecule are so different. Cleansing action of soaps and detergents. Updated on july 19, 2019. The reaction is called saponification and produces. Previously, the alkali which was. Soaps Are Long Chain Of.
From www.aplustopper.com
Explain the Cleansing Action Of Soaps and Detergents A Plus Topper Soaps Are Long Chain Of Generally, soap is created from a long chain of carbon atoms that carry two hydrogen atoms. Cleansing action of soaps and detergents. Previously, the alkali which was needed to. Most of the dirt is oily in nature and oil does not dissolve in water. Updated on july 19, 2019. Each soap molecule has a long. Soap is made via a. Soaps Are Long Chain Of.
From www.shutterstock.com
Soap Soaps Sodium Potassium Salts Long Stock Vector (Royalty Free Soaps Are Long Chain Of The reaction is called saponification and produces. Note that each of these molecules has a nonpolar hydrocarbon chain,. Generally, soap is created from a long chain of carbon atoms that carry two hydrogen atoms. Most of the dirt is oily in nature and oil does not dissolve in water. The molecule of soap constitutes. Soaps are sodium or potassium fatty. Soaps Are Long Chain Of.
From historymeetsscience.blogspot.com
Tales of scientific journeys Soap making 101 Soaps Are Long Chain Of Cleansing action of soaps and detergents. They are surfactants (compounds that reduce the surface tension between a. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). Each soap molecule has a long. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Previously, the alkali. Soaps Are Long Chain Of.
From www.pinterest.com
Salt n Soap Supply chain model Supply chain model, Soap supplies Soaps Are Long Chain Of The molecule of soap constitutes. Generally, soap is created from a long chain of carbon atoms that carry two hydrogen atoms. They are surfactants (compounds that reduce the surface tension between a. Note that each of these molecules has a nonpolar hydrocarbon chain,. The most common examples of such compounds are soaps and detergents, four of which are shown below.. Soaps Are Long Chain Of.
From tangofscience.blog
The chemistry of soaps and why it matters A tang of science Soaps Are Long Chain Of Soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). Previously, the alkali which was needed to. The most common examples of such compounds are soaps and detergents, four of which are shown below. The reaction is called. Soaps Are Long Chain Of.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID1978873 Soaps Are Long Chain Of The most common examples of such compounds are soaps and detergents, four of which are shown below. Previously, the alkali which was needed to. Most of the dirt is oily in nature and oil does not dissolve in water. Cleansing action of soaps and detergents. Soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain. Soaps Are Long Chain Of.
From www.researchgate.net
(PDF) Effects of calcium soaps of longchain fatty acids on feedlot Soaps Are Long Chain Of Soaps act as cleansers because the two ends of a soap molecule are so different. Note that each of these molecules has a nonpolar hydrocarbon chain,. The most common examples of such compounds are soaps and detergents, four of which are shown below. The molecule of soap constitutes. Soaps are potassium or sodium salts of a carboxylic acid having a. Soaps Are Long Chain Of.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Soaps Are Long Chain Of Previously, the alkali which was needed to. They are surfactants (compounds that reduce the surface tension between a. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). Most of the dirt is oily in nature and oil does not dissolve in water. Generally, soap is created from a long chain of carbon atoms that carry. Soaps Are Long Chain Of.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID Soaps Are Long Chain Of Generally, soap is created from a long chain of carbon atoms that carry two hydrogen atoms. Most of the dirt is oily in nature and oil does not dissolve in water. The reaction is called saponification and produces. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soaps are. Soaps Are Long Chain Of.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID Soaps Are Long Chain Of Each soap molecule has a long. Note that each of these molecules has a nonpolar hydrocarbon chain,. Previously, the alkali which was needed to. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). Updated on july 19, 2019. The reaction is called saponification and produces. Soaps act as cleansers because the two ends of a. Soaps Are Long Chain Of.
From www.researchgate.net
Cosmetics and soaps value chain Download Scientific Diagram Soaps Are Long Chain Of Each soap molecule has a long. Generally, soap is created from a long chain of carbon atoms that carry two hydrogen atoms. Soaps act as cleansers because the two ends of a soap molecule are so different. Cleansing action of soaps and detergents. They are surfactants (compounds that reduce the surface tension between a. Previously, the alkali which was needed. Soaps Are Long Chain Of.
From www.chegg.com
Solved Many household soaps are amphiphilic, often the salts Soaps Are Long Chain Of Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). Previously, the alkali which was needed to. Each soap molecule has a long. They are surfactants (compounds that reduce the surface tension between a. Most of the dirt is oily in nature and oil does not dissolve in water. Generally, soap is created from a long. Soaps Are Long Chain Of.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID Soaps Are Long Chain Of They are surfactants (compounds that reduce the surface tension between a. Each soap molecule has a long. Most of the dirt is oily in nature and oil does not dissolve in water. Soaps act as cleansers because the two ends of a soap molecule are so different. Generally, soap is created from a long chain of carbon atoms that carry. Soaps Are Long Chain Of.
From www.scribd.com
Soaps Are Sodium or Pottasium Salts of LongChain Fatty Acid. Structure Soaps Are Long Chain Of Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). They are surfactants (compounds that reduce the surface tension between a. Each soap molecule has a long. Cleansing action of soaps and detergents. The reaction is called saponification and produces. The molecule of soap constitutes. Most of the dirt is oily in nature and oil does. Soaps Are Long Chain Of.
From www.slideserve.com
PPT Soap Describe how soap is made from fatty acids and alkalis Soaps Are Long Chain Of Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). Updated on july 19, 2019. The reaction is called saponification and produces. The most common examples of such compounds are soaps and detergents, four of which are shown below. They are surfactants (compounds that reduce the surface tension between a. The molecule of soap constitutes. Soaps. Soaps Are Long Chain Of.
From www.scribd.com
Foaming Capacity of Soaps Long Hydrocarbon Chain Hydrophobic end Soaps Are Long Chain Of Generally, soap is created from a long chain of carbon atoms that carry two hydrogen atoms. They are surfactants (compounds that reduce the surface tension between a. Note that each of these molecules has a nonpolar hydrocarbon chain,. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). Soaps are sodium or potassium fatty acids salts,. Soaps Are Long Chain Of.
From slideplayer.com
SOAPS AND DETERGENTS. ppt download Soaps Are Long Chain Of The reaction is called saponification and produces. Soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. Updated on july 19, 2019. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). Generally, soap is created from a long chain of carbon atoms that carry two hydrogen atoms.. Soaps Are Long Chain Of.
From chemistry.about.com
What Is Soap Made of and How Does It Clean? Soaps Are Long Chain Of Soaps act as cleansers because the two ends of a soap molecule are so different. The most common examples of such compounds are soaps and detergents, four of which are shown below. The reaction is called saponification and produces. Soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. The molecule of. Soaps Are Long Chain Of.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID Soaps Are Long Chain Of The molecule of soap constitutes. They are surfactants (compounds that reduce the surface tension between a. Previously, the alkali which was needed to. Updated on july 19, 2019. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Most of the dirt is oily in nature and oil does not. Soaps Are Long Chain Of.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID1978873 Soaps Are Long Chain Of They are surfactants (compounds that reduce the surface tension between a. Previously, the alkali which was needed to. Generally, soap is created from a long chain of carbon atoms that carry two hydrogen atoms. Cleansing action of soaps and detergents. Soaps act as cleansers because the two ends of a soap molecule are so different. Updated on july 19, 2019.. Soaps Are Long Chain Of.
From www.chegg.com
Mixtures Of Esters Of Longchain Carboxylic Acids Soaps Are Long Chain Of Cleansing action of soaps and detergents. Previously, the alkali which was needed to. Soaps act as cleansers because the two ends of a soap molecule are so different. Soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. Each soap molecule has a long. Note that each of these molecules has a. Soaps Are Long Chain Of.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID3090261 Soaps Are Long Chain Of Note that each of these molecules has a nonpolar hydrocarbon chain,. They are surfactants (compounds that reduce the surface tension between a. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). Each soap molecule has a long. Most of the dirt is oily in nature and oil does not dissolve in water. Previously, the alkali. Soaps Are Long Chain Of.
From www.slideserve.com
PPT Soap Describe how soap is made from fatty acids and alkalis Soaps Are Long Chain Of Updated on july 19, 2019. Cleansing action of soaps and detergents. Most of the dirt is oily in nature and oil does not dissolve in water. Each soap molecule has a long. They are surfactants (compounds that reduce the surface tension between a. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). The most common. Soaps Are Long Chain Of.
From www.youtube.com
Types of Soap, Chemistry Lecture Sabaq.pk YouTube Soaps Are Long Chain Of Previously, the alkali which was needed to. Soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. The most common examples of such compounds are soaps and detergents, four of which are shown below. They are surfactants (compounds that reduce the surface tension between a. Note that each of these molecules has. Soaps Are Long Chain Of.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID Soaps Are Long Chain Of The reaction is called saponification and produces. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). The most common examples of such compounds are soaps and detergents, four of which are shown below. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soaps are. Soaps Are Long Chain Of.
From pubs.rsc.org
Part II. Special and technical. A. Soaps and other long chain Soaps Are Long Chain Of Previously, the alkali which was needed to. Generally, soap is created from a long chain of carbon atoms that carry two hydrogen atoms. The reaction is called saponification and produces. The most common examples of such compounds are soaps and detergents, four of which are shown below. The molecule of soap constitutes. Cleansing action of soaps and detergents. Each soap. Soaps Are Long Chain Of.
From www.slideserve.com
PPT Preparation and Properties of a Soap PowerPoint Presentation Soaps Are Long Chain Of Cleansing action of soaps and detergents. Each soap molecule has a long. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Updated on july 19, 2019. Soap is made via a chemical reaction between a fat and sodium hydroxide (naoh). Soaps are potassium or sodium salts of a carboxylic. Soaps Are Long Chain Of.