What Is Heat Capacity Chemistry Definition . Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Heat capacity is a measure of the amount of energy required to raise the temperature of a substance by one degree. Heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. It plays a crucial role in understanding. It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat.
from askfilo.com
It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. It is usually expressed as calories per degree in. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity is a measure of the amount of energy required to raise the temperature of a substance by one degree. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount.
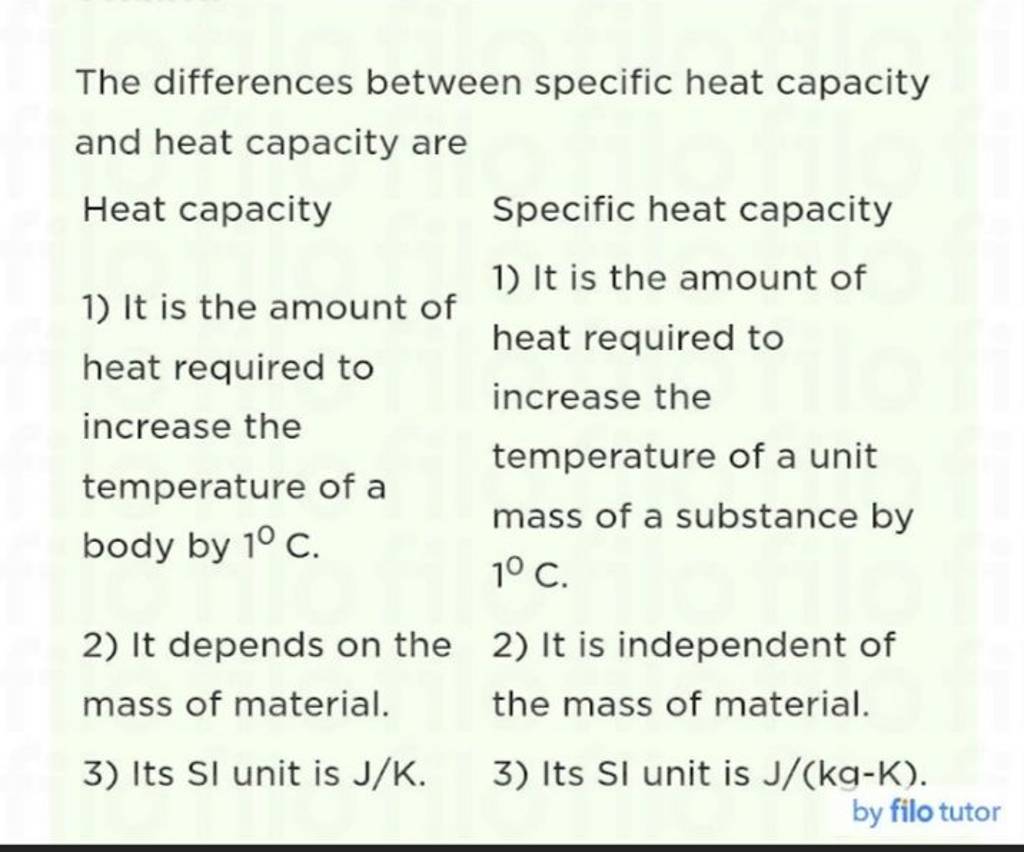
The differences between specific heat capacity and heat capacity are..
What Is Heat Capacity Chemistry Definition The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. It plays a crucial role in understanding. Heat capacity is a measure of the amount of energy required to raise the temperature of a substance by one degree. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change.
From www.differencebetween.com
Difference Between Heat Capacity and Specific Heat Compare the What Is Heat Capacity Chemistry Definition Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change. It is usually expressed as calories per degree in. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Heat capacity, ratio. What Is Heat Capacity Chemistry Definition.
From www.slideserve.com
PPT Chapter 17 Thermochemistry PowerPoint Presentation, free What Is Heat Capacity Chemistry Definition Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It plays a crucial role in understanding. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs or. What Is Heat Capacity Chemistry Definition.
From www.grc.nasa.gov
Heat Transfer What Is Heat Capacity Chemistry Definition It plays a crucial role in understanding. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change. It is usually expressed as calories per degree in. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is. What Is Heat Capacity Chemistry Definition.
From www.slideserve.com
PPT Properties of Materials PowerPoint Presentation, free download What Is Heat Capacity Chemistry Definition The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. It plays a crucial role in understanding. Heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. Heat capacity, ratio of heat absorbed by a material. What Is Heat Capacity Chemistry Definition.
From www.youtube.com
Chemistry 10.2 Specific Heat Capacity YouTube What Is Heat Capacity Chemistry Definition It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity is a measure of the amount of energy required to raise the temperature of a substance by one degree. Specific heat is defined as the amount. What Is Heat Capacity Chemistry Definition.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. What Is Heat Capacity Chemistry Definition Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity is a measure of the amount of energy. What Is Heat Capacity Chemistry Definition.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is Heat Capacity Chemistry Definition Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change. It is usually expressed as calories per degree in. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. The heat capacity. What Is Heat Capacity Chemistry Definition.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube What Is Heat Capacity Chemistry Definition Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It plays a crucial role in understanding. It is usually expressed as calories per degree in. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is. What Is Heat Capacity Chemistry Definition.
From www.youtube.com
Heat Capacity and Specific Heat Capacity Chemistry with Dr. G YouTube What Is Heat Capacity Chemistry Definition Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change. The heat capacity of a substance describes how its temperature. What Is Heat Capacity Chemistry Definition.
From www.slideserve.com
PPT Advance Chemical Engineering Thermodynamics PowerPoint What Is Heat Capacity Chemistry Definition Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is a measure of the amount of energy required to raise the temperature of a substance by one degree. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Specific. What Is Heat Capacity Chemistry Definition.
From brainly.in
difference between specific heat and heat capacity Brainly.in What Is Heat Capacity Chemistry Definition Heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories per degree in. Heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat. What Is Heat Capacity Chemistry Definition.
From slideshare.net
Heat Capacity What Is Heat Capacity Chemistry Definition It is usually expressed as calories per degree in. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. Heat capacity, also known as thermal capacity, is a. What Is Heat Capacity Chemistry Definition.
From education-portal.com
Latent Heat Definition, Formula & Examples Video & Lesson Transcript What Is Heat Capacity Chemistry Definition It is usually expressed as calories per degree in. Heat capacity is a measure of the amount of energy required to raise the temperature of a substance by one degree. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change. Heat capacity, ratio of heat. What Is Heat Capacity Chemistry Definition.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is Heat Capacity Chemistry Definition The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. It is usually expressed as calories per degree in. Heat capacity, also. What Is Heat Capacity Chemistry Definition.
From studylib.net
1.3 Specific Heat Capacity What Is Heat Capacity Chemistry Definition Heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories per degree in. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. The heat capacity of a substance describes how its temperature changes as it. What Is Heat Capacity Chemistry Definition.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is Heat Capacity Chemistry Definition Heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. It is usually expressed as calories per degree in. Heat capacity is a measure of the amount of energy required to raise the temperature of a substance by one degree. Heat capacity, ratio of heat absorbed by a material to the. What Is Heat Capacity Chemistry Definition.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Is Heat Capacity Chemistry Definition Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. Heat capacity is a measure of the amount of energy required to raise the temperature of a substance by one degree. Heat capacity, also known as thermal capacity,. What Is Heat Capacity Chemistry Definition.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is Heat Capacity Chemistry Definition It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. The heat capacity of a substance describes how. What Is Heat Capacity Chemistry Definition.
From www.slideserve.com
PPT THERMOCHEMISTRY Thermodynamics The study of Heat and Work and What Is Heat Capacity Chemistry Definition It plays a crucial role in understanding. Heat capacity is a measure of the amount of energy required to raise the temperature of a substance by one degree. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity is the amount of heat. What Is Heat Capacity Chemistry Definition.
From www.geeksforgeeks.org
Heat Capacity Definition, Formula, Unit, Examples, FAQs What Is Heat Capacity Chemistry Definition Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. It is usually expressed as calories per degree in. Heat capacity is the amount of heat energy required to raise the temperature. What Is Heat Capacity Chemistry Definition.
From www.youtube.com
Specific heat capacity YouTube What Is Heat Capacity Chemistry Definition Heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity, also. What Is Heat Capacity Chemistry Definition.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii What Is Heat Capacity Chemistry Definition Heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories per degree in. Heat capacity is a measure of the amount of energy required to raise the temperature of a substance by one degree. Heat capacity is the amount of heat energy required to raise the temperature of a body a. What Is Heat Capacity Chemistry Definition.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Heat Capacity Chemistry Definition It is usually expressed as calories per degree in. Heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. It plays a crucial role in understanding. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change.. What Is Heat Capacity Chemistry Definition.
From www.slideserve.com
PPT Thermochemistry The heat energy of chemical reactions PowerPoint What Is Heat Capacity Chemistry Definition It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs or. What Is Heat Capacity Chemistry Definition.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Is Heat Capacity Chemistry Definition The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity is a measure of the amount of energy required to raise the temperature of a substance by one degree. Heat capacity, also known as thermal capacity, is a physical property of matter defined. What Is Heat Capacity Chemistry Definition.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems What Is Heat Capacity Chemistry Definition Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. The heat capacity of a substance describes how its temperature changes as. What Is Heat Capacity Chemistry Definition.
From studylib.net
Heat Equation What Is Heat Capacity Chemistry Definition Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of. What Is Heat Capacity Chemistry Definition.
From www.youtube.com
Heat Capacity and Specific Heat Chemistry Tutorial YouTube What Is Heat Capacity Chemistry Definition Heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. It plays a crucial role in understanding. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change. Specific heat is defined as the amount of heat. What Is Heat Capacity Chemistry Definition.
From gamesmartz.com
Heat Capacity Definition Easy to Understand What Is Heat Capacity Chemistry Definition The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. It is usually expressed as calories per degree in. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Heat capacity, ratio. What Is Heat Capacity Chemistry Definition.
From studymind.co.uk
Heat Capacity Study Mind What Is Heat Capacity Chemistry Definition It is usually expressed as calories per degree in. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a. What Is Heat Capacity Chemistry Definition.
From www.youtube.com
Heat Capacity and Specific Heat YouTube What Is Heat Capacity Chemistry Definition Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change. Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by. What Is Heat Capacity Chemistry Definition.
From thechemistrynotes.com
Heat Capacity and Specific Heat What Is Heat Capacity Chemistry Definition Heat capacity is a measure of the amount of energy required to raise the temperature of a substance by one degree. It is usually expressed as calories per degree in. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change. Heat capacity is the amount. What Is Heat Capacity Chemistry Definition.
From scienceinfo.com
Specific Heat Capacity Definition, Unit, Formula What Is Heat Capacity Chemistry Definition Heat capacity is a measure of the amount of energy required to raise the temperature of a substance by one degree. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. The heat capacity of a substance describes how its temperature changes as it absorbs. What Is Heat Capacity Chemistry Definition.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity What Is Heat Capacity Chemistry Definition Heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity, ratio of heat absorbed by a material. What Is Heat Capacity Chemistry Definition.
From www.pinterest.com
Specific Heat Easy Science Teaching chemistry, Sensible heat What Is Heat Capacity Chemistry Definition Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. Specific heat is defined as. What Is Heat Capacity Chemistry Definition.