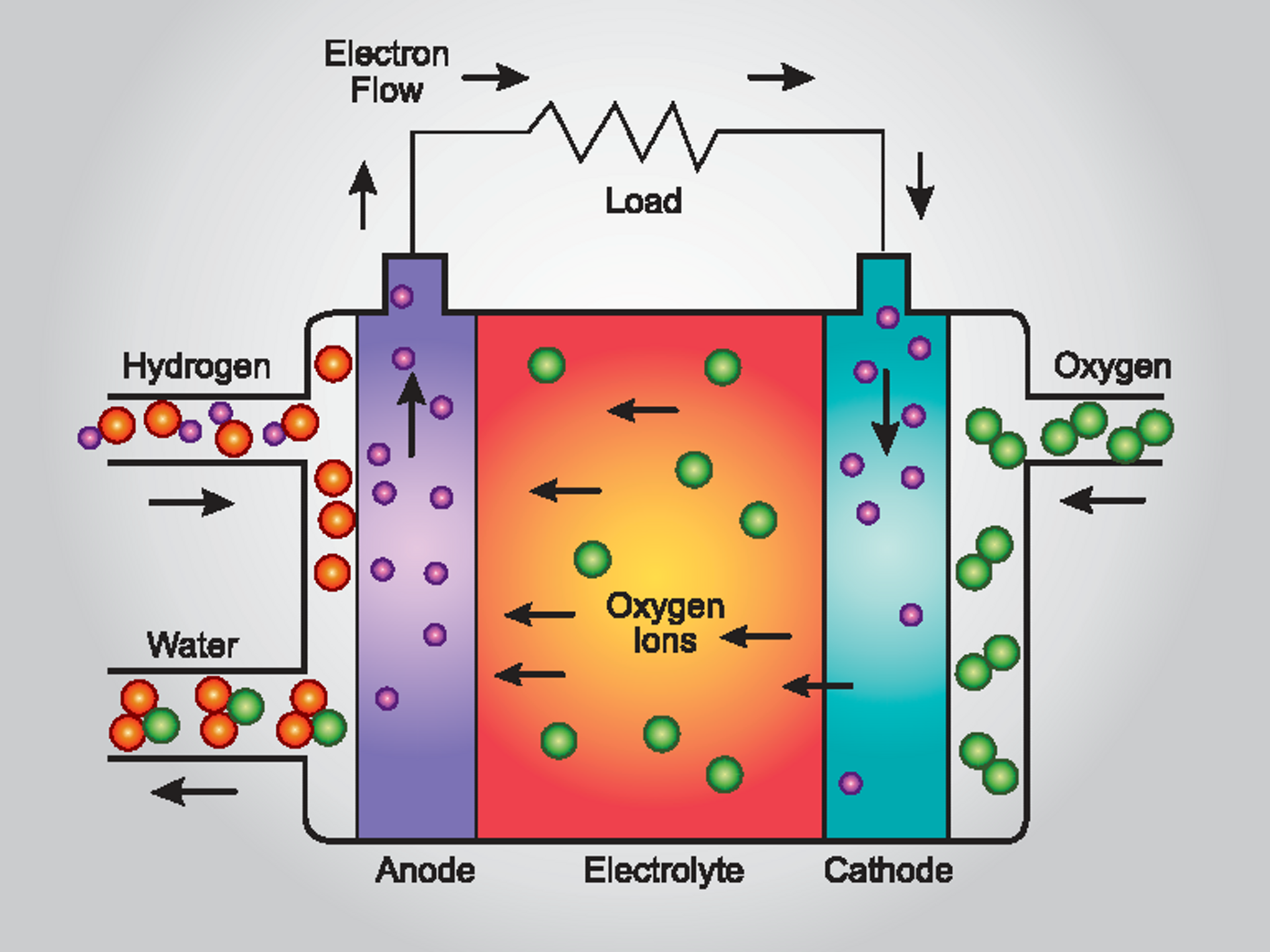
How Do Hydrogen Fuel Cells Work A Level Chemistry . Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Hydrogen, for example, reacts spontaneously with oxygen with the high energy content of the oxidation of hydrogen being completely released as heat. This is done when an electrochemical reaction takes place between hydrogen fuel and oxygen or any other oxidizing agent. In a fuel cell the fuel does not react directly with the oxidant, but instead delivers its electrons to the anode. Electrochemical cells generate electric current through electrode reactions. Fuel cells do not require. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. These cells can be batteries or fuel cells. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to a similar electrode except that the catalyst is silver. While in batteries chemical energy is generated from the chemicals that are already filled in it, fuel cells need a. At the anode, hydrogen gas. Diagram showing the movement of.
from www.air101.co.uk
Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Fuel cells do not require. Electrochemical cells generate electric current through electrode reactions. These cells can be batteries or fuel cells. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. While in batteries chemical energy is generated from the chemicals that are already filled in it, fuel cells need a. Diagram showing the movement of. Hydrogen, for example, reacts spontaneously with oxygen with the high energy content of the oxidation of hydrogen being completely released as heat. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to.
Air101 Hydrogen fuel cells, explained
How Do Hydrogen Fuel Cells Work A Level Chemistry Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Hydrogen, for example, reacts spontaneously with oxygen with the high energy content of the oxidation of hydrogen being completely released as heat. These cells can be batteries or fuel cells. This is done when an electrochemical reaction takes place between hydrogen fuel and oxygen or any other oxidizing agent. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. While in batteries chemical energy is generated from the chemicals that are already filled in it, fuel cells need a. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Oxygen is supplied to a similar electrode except that the catalyst is silver. Diagram showing the movement of. Fuel cells do not require. At the anode, hydrogen gas. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Electrochemical cells generate electric current through electrode reactions. In a fuel cell the fuel does not react directly with the oxidant, but instead delivers its electrons to the anode.
From www.youtube.com
How hydrogen fuel cell works Fuel Cell Technology Working principle How Do Hydrogen Fuel Cells Work A Level Chemistry These cells can be batteries or fuel cells. This is done when an electrochemical reaction takes place between hydrogen fuel and oxygen or any other oxidizing agent. In a fuel cell the fuel does not react directly with the oxidant, but instead delivers its electrons to the anode. At the anode, hydrogen gas. While in batteries chemical energy is generated. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记6.1.8 Fuel Cells翰林国际教育 How Do Hydrogen Fuel Cells Work A Level Chemistry The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. These cells can be batteries or fuel cells. At the anode, hydrogen gas. In a fuel cell the fuel does not react directly with the oxidant, but instead delivers its electrons to the anode. Electrochemical cells. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working How Do Hydrogen Fuel Cells Work A Level Chemistry This is done when an electrochemical reaction takes place between hydrogen fuel and oxygen or any other oxidizing agent. Fuel cells do not require. Oxygen is supplied to a similar electrode except that the catalyst is silver. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Typically, in a hydrogen fuel cell,. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From ar.inspiredpencil.com
How Hydrogen Fuel Cells Work How Do Hydrogen Fuel Cells Work A Level Chemistry While in batteries chemical energy is generated from the chemicals that are already filled in it, fuel cells need a. Electrochemical cells generate electric current through electrode reactions. In a fuel cell the fuel does not react directly with the oxidant, but instead delivers its electrons to the anode. Fuel cells do not require. Hydrogen enters the cell through a. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.dreamstime.com
Hydrogen Fuel Cells. Education Infographic. Vector Design. Stock Vector How Do Hydrogen Fuel Cells Work A Level Chemistry Fuel cells do not require. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. At the anode, hydrogen gas. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. This is done when an electrochemical reaction takes place between hydrogen fuel. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From exyueugsn.blob.core.windows.net
Do All Fuel Cells Use Hydrogen at Donald Venable blog How Do Hydrogen Fuel Cells Work A Level Chemistry These cells can be batteries or fuel cells. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Electrochemical cells generate electric current through electrode reactions. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. Typically, in a hydrogen fuel. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica How Do Hydrogen Fuel Cells Work A Level Chemistry Oxygen is supplied to a similar electrode except that the catalyst is silver. At the anode, hydrogen gas. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Fuel cells do not require. Fuel. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From large.stanford.edu
Hydrogen Fuel Cells How Do Hydrogen Fuel Cells Work A Level Chemistry Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Fuel cells do not require. Electrochemical cells generate electric current through electrode reactions. The most common type of fuel cell is the hydrogen fuel. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From exyamfqge.blob.core.windows.net
Advantages Of Hydrogen Fuel Cells Gcse Chemistry at Clinton Earl blog How Do Hydrogen Fuel Cells Work A Level Chemistry Hydrogen, for example, reacts spontaneously with oxygen with the high energy content of the oxidation of hydrogen being completely released as heat. These cells can be batteries or fuel cells. Oxygen is supplied to a similar electrode except that the catalyst is silver. Fuel cells do not require. The most common type of fuel cell is the hydrogen fuel cell,. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.youtube.com
How does a hydrogen fuel cell work? (AKIO TV) YouTube How Do Hydrogen Fuel Cells Work A Level Chemistry Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. At the anode, hydrogen gas. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From courses.lumenlearning.com
Phases and Classification of Matter Introductory Chemistry Lecture How Do Hydrogen Fuel Cells Work A Level Chemistry The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. Electrochemical cells generate electric current through electrode reactions. Diagram showing the movement of. Hydrogen, for example, reacts spontaneously with oxygen with the high energy content of the oxidation of hydrogen being completely released as heat. Fuel. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.sukorun.com
Hydrogen Fuel Cells Q&A Part 1 Sukorun How Do Hydrogen Fuel Cells Work A Level Chemistry Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. At the anode, hydrogen gas. Fuel cells do not require. Electrochemical cells generate electric current through electrode reactions. In a fuel cell the fuel does not react directly with the oxidant, but instead delivers its electrons to the anode.. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.researchgate.net
Schematic diagram of hydrogen fuel cell Download Scientific Diagram How Do Hydrogen Fuel Cells Work A Level Chemistry Fuel cells do not require. Oxygen is supplied to a similar electrode except that the catalyst is silver. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK How Do Hydrogen Fuel Cells Work A Level Chemistry Diagram showing the movement of. This is done when an electrochemical reaction takes place between hydrogen fuel and oxygen or any other oxidizing agent. While in batteries chemical energy is generated from the chemicals that are already filled in it, fuel cells need a. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. These. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From powertorque.com.au
Cummins Hydrogen FUEL CELL infographic_ Power Torque How Do Hydrogen Fuel Cells Work A Level Chemistry Diagram showing the movement of. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. Fuel cells do not require. Oxygen is supplied to a similar electrode except that the catalyst is silver. This is done when an electrochemical reaction takes place between hydrogen fuel and. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.researchgate.net
6. Hydrogenoxygen fuel cell scheme. Download Scientific Diagram How Do Hydrogen Fuel Cells Work A Level Chemistry While in batteries chemical energy is generated from the chemicals that are already filled in it, fuel cells need a. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The most common type of fuel cell is. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.alamy.com
How fuel cells work to produce electricity from hydrogen and air Stock How Do Hydrogen Fuel Cells Work A Level Chemistry While in batteries chemical energy is generated from the chemicals that are already filled in it, fuel cells need a. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Fuel cells do not require. Hydrogen, for example, reacts spontaneously with oxygen with the high energy content of the oxidation of hydrogen being completely released. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.air101.co.uk
Air101 Hydrogen fuel cells, explained How Do Hydrogen Fuel Cells Work A Level Chemistry Hydrogen, for example, reacts spontaneously with oxygen with the high energy content of the oxidation of hydrogen being completely released as heat. These cells can be batteries or fuel cells. In a fuel cell the fuel does not react directly with the oxidant, but instead delivers its electrons to the anode. While in batteries chemical energy is generated from the. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.studypool.com
SOLUTION Fuel cells,construction ,working and examples Studypool How Do Hydrogen Fuel Cells Work A Level Chemistry Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. These cells can be batteries or fuel cells. Hydrogen, for example, reacts spontaneously with oxygen with the high energy content of the oxidation of hydrogen being completely released as heat. Fuel cells do not require. In a fuel cell the fuel does not react directly. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From chem.libretexts.org
6.7 Batteries and Fuel Cells Chemistry LibreTexts How Do Hydrogen Fuel Cells Work A Level Chemistry In a fuel cell the fuel does not react directly with the oxidant, but instead delivers its electrons to the anode. Fuel cells do not require. Oxygen is supplied to a similar electrode except that the catalyst is silver. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.linquip.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip How Do Hydrogen Fuel Cells Work A Level Chemistry Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Electrochemical cells generate electric current through electrode reactions. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. These cells can be batteries or fuel cells. At the anode, hydrogen gas. This is done when an electrochemical reaction. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.setra.com
What is a Hydrogen Fuel Cell? How Do Hydrogen Fuel Cells Work A Level Chemistry Diagram showing the movement of. Electrochemical cells generate electric current through electrode reactions. While in batteries chemical energy is generated from the chemicals that are already filled in it, fuel cells need a. Hydrogen, for example, reacts spontaneously with oxygen with the high energy content of the oxidation of hydrogen being completely released as heat. Fuel cells do not require.. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.instructables.com
How to Make a Simple Hydrogen Fuel Cell 8 Steps Instructables How Do Hydrogen Fuel Cells Work A Level Chemistry Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Hydrogen, for example, reacts spontaneously with oxygen with the high energy content of the oxidation of hydrogen being completely released as heat. Fuel cells. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.chfca.ca
About Fuel Cells CHFCA How Do Hydrogen Fuel Cells Work A Level Chemistry Oxygen is supplied to a similar electrode except that the catalyst is silver. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Hydrogen, for. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.sundyne.com
Hydrogen Fuel Cells How Do They Work? Sundyne How Do Hydrogen Fuel Cells Work A Level Chemistry Electrochemical cells generate electric current through electrode reactions. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Oxygen is supplied to a similar electrode except that the catalyst is silver. These cells can be batteries or fuel cells. This is done when an electrochemical reaction takes place between. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From exoahaoro.blob.core.windows.net
Fuel Cell Chemistry And Operation at Russell Lane blog How Do Hydrogen Fuel Cells Work A Level Chemistry This is done when an electrochemical reaction takes place between hydrogen fuel and oxygen or any other oxidizing agent. Electrochemical cells generate electric current through electrode reactions. Oxygen is supplied to a similar electrode except that the catalyst is silver. While in batteries chemical energy is generated from the chemicals that are already filled in it, fuel cells need a.. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.researchgate.net
e Schematic view of a typical hydrogen PEM fuel cell and its components How Do Hydrogen Fuel Cells Work A Level Chemistry Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Diagram showing the movement of. Fuel cells do not require. While in batteries chemical energy is generated from the chemicals that are already filled in it, fuel cells need a. Oxygen is supplied to a similar electrode except that the catalyst is silver. Typically, in. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From energytheory.com
Hydrogen Fuel Cells Explained Efficiency and Working Energy Theory How Do Hydrogen Fuel Cells Work A Level Chemistry Hydrogen, for example, reacts spontaneously with oxygen with the high energy content of the oxidation of hydrogen being completely released as heat. While in batteries chemical energy is generated from the chemicals that are already filled in it, fuel cells need a. At the anode, hydrogen gas. The most common type of fuel cell is the hydrogen fuel cell, which. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From large.stanford.edu
Hydrogen Evolution Reaction How Do Hydrogen Fuel Cells Work A Level Chemistry Fuel cells do not require. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. In a fuel cell the fuel does not react directly with the oxidant, but instead delivers its electrons to the anode. These cells can be batteries or fuel cells. Electrochemical cells. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.simscale.com
Hydrogen Fuel Cell Simulation & Modeling Blog SimScale How Do Hydrogen Fuel Cells Work A Level Chemistry Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. Diagram showing the movement of. While in batteries chemical energy is generated from the chemicals. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From www.thestudentroom.co.uk
Hydrogen fuel cells The Student Room How Do Hydrogen Fuel Cells Work A Level Chemistry At the anode, hydrogen gas. This is done when an electrochemical reaction takes place between hydrogen fuel and oxygen or any other oxidizing agent. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. Diagram showing the movement of. Hydrogen enters the cell through a porous. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From totalshield.com
Electrolyzer and Hydrogen Fuel Cell Safety TotalShield How Do Hydrogen Fuel Cells Work A Level Chemistry Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Fuel cells do not require. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. In a fuel cell the fuel does not react directly. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From mavink.com
Hydrogen Fuel Cell Process How Do Hydrogen Fuel Cells Work A Level Chemistry Oxygen is supplied to a similar electrode except that the catalyst is silver. These cells can be batteries or fuel cells. In a fuel cell the fuel does not react directly with the oxidant, but instead delivers its electrons to the anode. The most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From guidewiringdietician.z5.web.core.windows.net
Fuel Cell Diagram Gcse How Do Hydrogen Fuel Cells Work A Level Chemistry While in batteries chemical energy is generated from the chemicals that are already filled in it, fuel cells need a. This is done when an electrochemical reaction takes place between hydrogen fuel and oxygen or any other oxidizing agent. At the anode, hydrogen gas. Fuel cells do not require. Hydrogen, for example, reacts spontaneously with oxygen with the high energy. How Do Hydrogen Fuel Cells Work A Level Chemistry.
From mungfali.com
Hydrogen Fuel Cell Chart How Do Hydrogen Fuel Cells Work A Level Chemistry In a fuel cell the fuel does not react directly with the oxidant, but instead delivers its electrons to the anode. Diagram showing the movement of. While in batteries chemical energy is generated from the chemicals that are already filled in it, fuel cells need a. These cells can be batteries or fuel cells. Oxygen is supplied to a similar. How Do Hydrogen Fuel Cells Work A Level Chemistry.