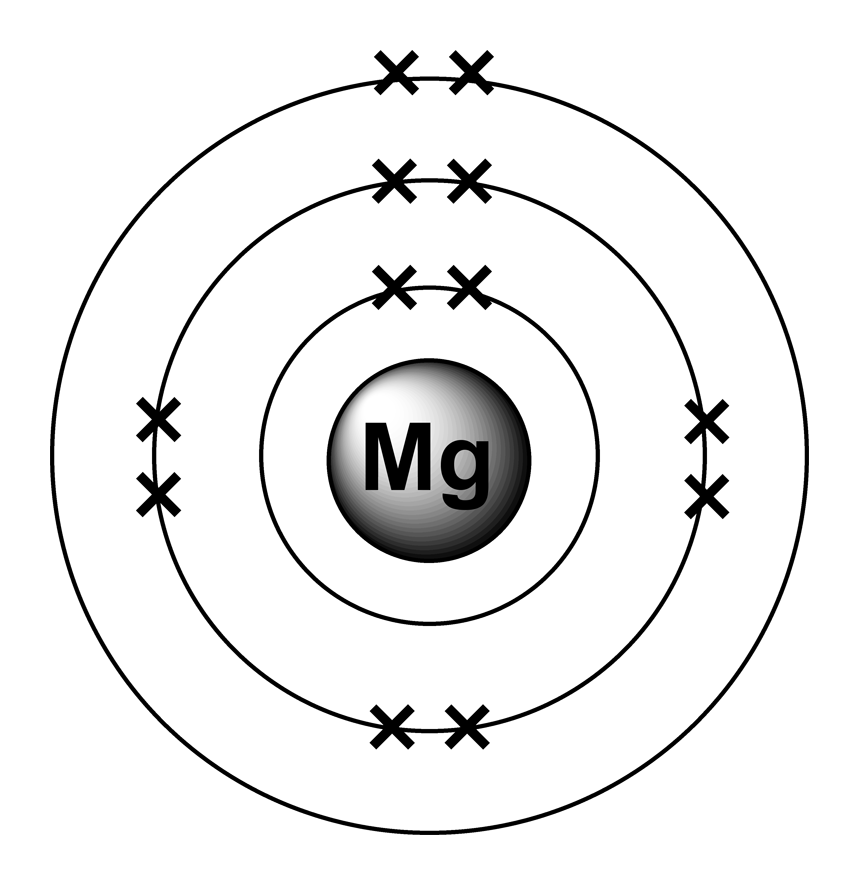
Magnesium Ion Energy Level Diagram . The order is summarized under. Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels. The ground state electron configuration of magnesium is important in understanding its chemical properties. Electrons in lower energy levels are called the core electrons. To use this figure, read along the diagonal lines in the direction of the arrow. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Bohr diagrams indicate how many electrons fill each principal shell. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The principal energy levels are listed in columns, starting at the left with the 1s level. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. Magnesium has 12 electrons arranged in three energy levels.
from www.animalia-life.club
The principal energy levels are listed in columns, starting at the left with the 1s level. Electrons in lower energy levels are called the core electrons. Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels. Bohr diagrams indicate how many electrons fill each principal shell. The ground state electron configuration of magnesium is important in understanding its chemical properties. The order is summarized under. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell.
Magnesium Electron Configuration
Magnesium Ion Energy Level Diagram Bohr diagrams indicate how many electrons fill each principal shell. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Bohr diagrams indicate how many electrons fill each principal shell. To use this figure, read along the diagonal lines in the direction of the arrow. The order is summarized under. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The ground state electron configuration of magnesium is important in understanding its chemical properties. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. Electrons in lower energy levels are called the core electrons. The principal energy levels are listed in columns, starting at the left with the 1s level. Magnesium has 12 electrons arranged in three energy levels. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Energy Level Diagram Bohr diagrams indicate how many electrons fill each principal shell. The ground state electron configuration of magnesium is important in understanding its chemical properties. The principal energy levels are listed in columns, starting at the left with the 1s level. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Let's look at the figure below which shows the electron. Magnesium Ion Energy Level Diagram.
From mungfali.com
Magnesium Orbital Diagram Magnesium Ion Energy Level Diagram Bohr diagrams indicate how many electrons fill each principal shell. To use this figure, read along the diagonal lines in the direction of the arrow. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The principal energy levels are listed in columns,. Magnesium Ion Energy Level Diagram.
From www.myxxgirl.com
Atom Diagram Of Magnesium Diagram To Show Ionic Bonding In Magnesium Magnesium Ion Energy Level Diagram Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels. Electrons in lower energy levels are called the core electrons. The order is summarized under. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. The principal energy levels are listed in. Magnesium Ion Energy Level Diagram.
From www.nagwa.com
Question Video Identifying the Energy Level Diagram That Represents Magnesium Ion Energy Level Diagram Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a. Magnesium Ion Energy Level Diagram.
From www.dreamstime.com
Magnesium Atom, with Mass and Energy Levels. Stock Vector Magnesium Ion Energy Level Diagram The order is summarized under. The principal energy levels are listed in columns, starting at the left with the 1s level. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons.. Magnesium Ion Energy Level Diagram.
From www.alamy.com
Magnesium atom, with mass and energy levels. Vector illustration Stock Magnesium Ion Energy Level Diagram To use this figure, read along the diagonal lines in the direction of the arrow. Bohr diagrams indicate how many electrons fill each principal shell. Electrons in lower energy levels are called the core electrons. Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels. The principal energy. Magnesium Ion Energy Level Diagram.
From wind-power.buffalomountainkombucha.com
[DIAGRAM] Lewis Electron Dot Diagrams Magnesium Magnesium Ion Energy Level Diagram The order is summarized under. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. The ground state electron configuration of magnesium is important in understanding its chemical properties. Magnesium has 12 electrons arranged in three energy levels. Students will first look at a diagram and animation to understand the basic pattern of the. Magnesium Ion Energy Level Diagram.
From www.slideserve.com
PPT Orbital Diagrams PowerPoint Presentation ID6677860 Magnesium Ion Energy Level Diagram To use this figure, read along the diagonal lines in the direction of the arrow. Electrons in lower energy levels are called the core electrons. The order is summarized under. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. The ground state electron configuration of magnesium is. Magnesium Ion Energy Level Diagram.
From www.mikrora.com
File Electron Configuration Magnesium Svg Best Diagram Collection Magnesium Ion Energy Level Diagram The ground state electron configuration of magnesium is important in understanding its chemical properties. The order is summarized under. Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Group 18 elements (helium, neon, and argon are shown). Magnesium Ion Energy Level Diagram.
From www.youtube.com
Atomic Structure (Bohr Model) for Magnesium (Mg) YouTube Magnesium Ion Energy Level Diagram The principal energy levels are listed in columns, starting at the left with the 1s level. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. To use this figure, read along the diagonal lines in the direction of the arrow. The diagram of the magnesium ion shows a central magnesium atom with a. Magnesium Ion Energy Level Diagram.
From www.americanelements.com
Magnesium (Mg) AMERICAN ELEMENTSs Magnesium Ion Energy Level Diagram The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. The principal energy levels are listed in columns, starting at the left with the 1s level. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. Group 18 elements. Magnesium Ion Energy Level Diagram.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Ion Energy Level Diagram The ground state electron configuration of magnesium is important in understanding its chemical properties. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. Electrons in lower energy levels are called the core electrons. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Let's look at the. Magnesium Ion Energy Level Diagram.
From diagramlevel1-11.blogspot.com
48 ENERGY LEVEL DIAGRAM MAGNESIUM DiagramLevel Magnesium Ion Energy Level Diagram The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. The principal energy levels are listed in columns, starting at the left with the 1s level. Students will first. Magnesium Ion Energy Level Diagram.
From www.alamy.com
Diagram to show ionic bonding in magnesium oxide Stock Vector Image Magnesium Ion Energy Level Diagram The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. The principal energy levels are listed in columns, starting at the left with the 1s level. The ground state electron configuration of magnesium is important in understanding its chemical properties. To use this figure, read along the diagonal. Magnesium Ion Energy Level Diagram.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Ion Energy Level Diagram Magnesium has 12 electrons arranged in three energy levels. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels. The order is summarized under. Electrons in lower energy levels are called the core electrons. The principal energy levels. Magnesium Ion Energy Level Diagram.
From utedzz.blogspot.com
Periodic Table Magnesium Atomic Number Periodic Table Timeline Magnesium Ion Energy Level Diagram Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. The ground state electron configuration of magnesium is important in understanding its chemical properties. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The order is summarized under. Magnesium has 12 electrons arranged in three energy. Magnesium Ion Energy Level Diagram.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Ion Energy Level Diagram Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. The order is summarized under. Let's look at the figure below which shows the electron diagram. Magnesium Ion Energy Level Diagram.
From guidedehartsculptures.z21.web.core.windows.net
Magnesium Electric Dot Diagram Magnesium Ion Energy Level Diagram Magnesium has 12 electrons arranged in three energy levels. To use this figure, read along the diagonal lines in the direction of the arrow. The ground state electron configuration of magnesium is important in understanding its chemical properties. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Bohr diagrams indicate how many electrons fill each principal shell. The diagram. Magnesium Ion Energy Level Diagram.
From exoilgobw.blob.core.windows.net
Magnesium Ion Explanation at Helen Weeks blog Magnesium Ion Energy Level Diagram Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels. The ground state electron configuration of magnesium is important in understanding its chemical properties. Bohr diagrams indicate how many electrons fill each principal shell. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons. Magnesium Ion Energy Level Diagram.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Ion Energy Level Diagram Magnesium has 12 electrons arranged in three energy levels. The order is summarized under. The principal energy levels are listed in columns, starting at the left with the 1s level. Electrons in lower energy levels are called the core electrons. Bohr diagrams indicate how many electrons fill each principal shell. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².. Magnesium Ion Energy Level Diagram.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Magnesium Ion Energy Level Diagram Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels. The order is summarized under. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The ground state electron configuration of magnesium is important in understanding its chemical properties. Electrons in lower energy levels are called the core. Magnesium Ion Energy Level Diagram.
From www.coursehero.com
[Solved] 3. Draw energy level diagrams for beryllium, magnesium and Magnesium Ion Energy Level Diagram Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. The principal energy levels are. Magnesium Ion Energy Level Diagram.
From www.thesciencehive.co.uk
The Periodic Table (GCSE) — the science hive Magnesium Ion Energy Level Diagram The order is summarized under. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. To use this figure, read along the diagonal lines in the direction of the arrow. Electrons in lower energy levels are called the core electrons. The diagram of the magnesium ion shows a central magnesium atom with a charge. Magnesium Ion Energy Level Diagram.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion Energy Level Diagram The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The principal energy levels are listed in columns, starting at the left with the 1s level. The ground state electron configuration of magnesium is important in understanding its chemical properties. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud. Magnesium Ion Energy Level Diagram.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy Magnesium Ion Energy Level Diagram Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels. The order is summarized under. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Magnesium has 12 electrons arranged in. Magnesium Ion Energy Level Diagram.
From mungfali.com
Magnesium Ion Electron Configuration Magnesium Ion Energy Level Diagram Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The order is summarized under. The principal energy levels are listed in columns, starting at the left with the 1s level. Bohr diagrams indicate how many electrons fill each principal shell. The ground state electron configuration of magnesium is important in understanding its. Magnesium Ion Energy Level Diagram.
From www.sciencephoto.com
Magnesium, atomic structure Stock Image C013/1519 Science Photo Library Magnesium Ion Energy Level Diagram Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. The ground state electron configuration of magnesium is important in understanding its chemical properties. The principal energy levels are listed in columns, starting at the left with. Magnesium Ion Energy Level Diagram.
From www.researchgate.net
Energylevel diagram for magnesium showing the levels relevant for this Magnesium Ion Energy Level Diagram The ground state electron configuration of magnesium is important in understanding its chemical properties. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. To use this figure, read along the diagonal lines in the direction of the arrow. Magnesium has 12 electrons arranged. Magnesium Ion Energy Level Diagram.
From www.researchgate.net
Energylevel diagram for magnesium. The full length horizontal lines Magnesium Ion Energy Level Diagram Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. The principal energy levels are listed in columns, starting at the left with the 1s level. Bohr diagrams indicate how many electrons fill each principal shell. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The ground state electron configuration of magnesium is. Magnesium Ion Energy Level Diagram.
From www.researchgate.net
Energylevel diagram for magnesium showing the levels relevant for this Magnesium Ion Energy Level Diagram Electrons in lower energy levels are called the core electrons. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. To use this figure, read along the diagonal lines in the direction of the arrow. Bohr diagrams indicate how many electrons fill each. Magnesium Ion Energy Level Diagram.
From joizkfdrp.blob.core.windows.net
Magnesium Ion Aufbau Diagram at Katherine Cortez blog Magnesium Ion Energy Level Diagram The ground state electron configuration of magnesium is important in understanding its chemical properties. To use this figure, read along the diagonal lines in the direction of the arrow. Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels. The electron configuration of magnesium is 1s² 2s² 2p⁶. Magnesium Ion Energy Level Diagram.
From trueufiles204.weebly.com
Magnesium Valence Electrons trueufiles Magnesium Ion Energy Level Diagram Electrons in lower energy levels are called the core electrons. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Bohr diagrams indicate how many electrons fill. Magnesium Ion Energy Level Diagram.
From www.slideserve.com
PPT An Introduction to Optical Atomic Spectrometry PowerPoint Magnesium Ion Energy Level Diagram Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The order is summarized under. The principal energy levels are listed in columns, starting at the left with the 1s level. Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels.. Magnesium Ion Energy Level Diagram.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Energy Level Diagram Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Magnesium has 12 electrons arranged in three. Magnesium Ion Energy Level Diagram.
From celbncbc.blob.core.windows.net
Magnesium Ion Number Of Electrons at Joan Harbert blog Magnesium Ion Energy Level Diagram Bohr diagrams indicate how many electrons fill each principal shell. To use this figure, read along the diagonal lines in the direction of the arrow. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. The ground state electron configuration of magnesium is important in understanding its chemical. Magnesium Ion Energy Level Diagram.