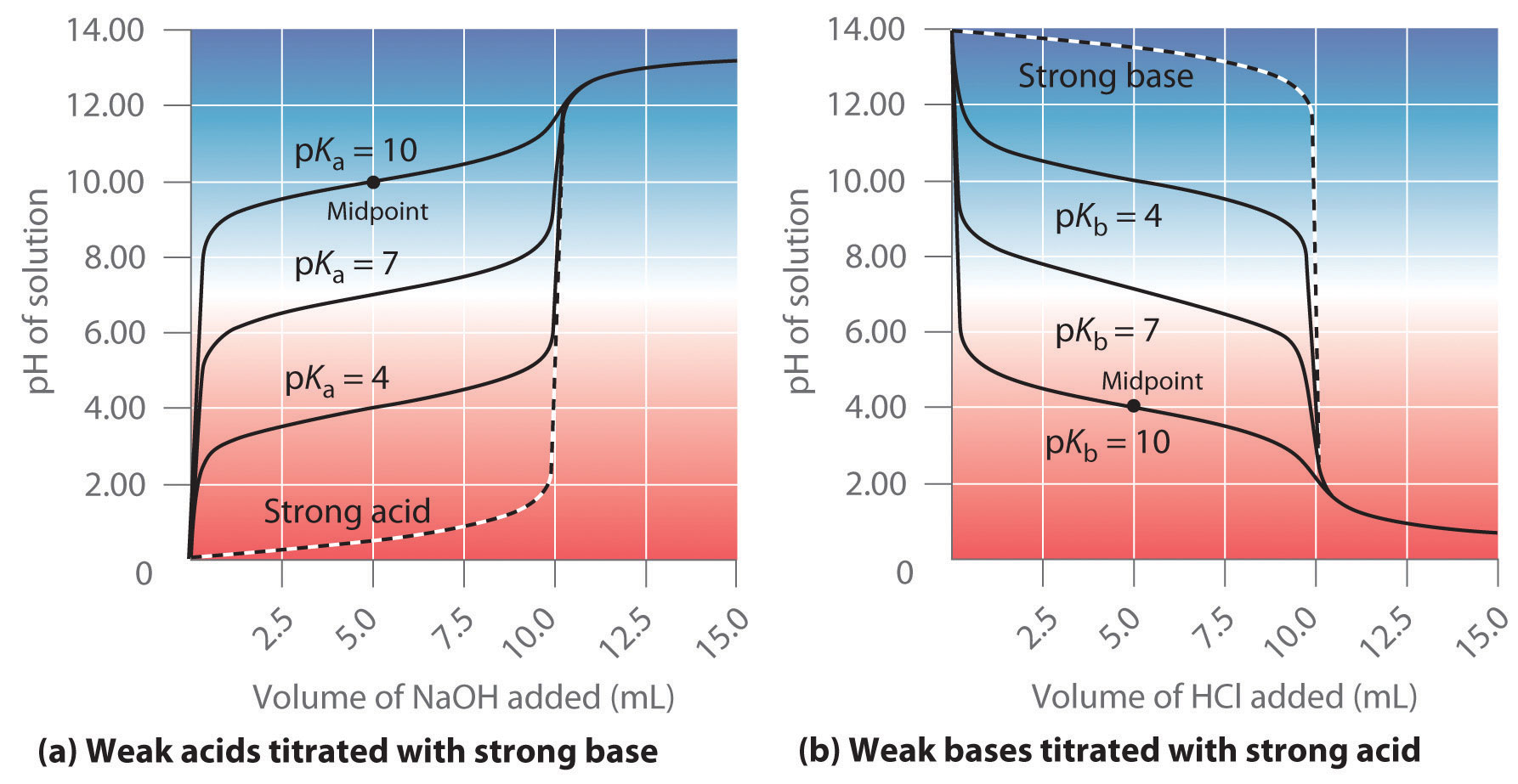
Titration Curve With Strong Acid And Strong Base . The reaction of the weak acid, acetic. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. the ph curve diagram below represents the titration of a strong acid with a strong base: Compute sample ph at important stages of a titration. titration curves for strong acid v strong base. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: As we add strong base to a strong acid,.
from saylordotorg.github.io
The reaction of the weak acid, acetic. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. As we add strong base to a strong acid,. Compute sample ph at important stages of a titration. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: titration curves for strong acid v strong base. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the.
AcidBase Titrations
Titration Curve With Strong Acid And Strong Base the ph curve diagram below represents the titration of a strong acid with a strong base: titration curves for strong acid v strong base. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. Compute sample ph at important stages of a titration. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. As we add strong base to a strong acid,. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. The reaction of the weak acid, acetic. the ph curve diagram below represents the titration of a strong acid with a strong base: Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the.
From mungfali.com
Strong Acid And Base Titration Curve Titration Curve With Strong Acid And Strong Base We'll take hydrochloric acid and sodium hydroxide as typical of a strong. titration curves for strong acid v strong base. Compute sample ph at important stages of a titration. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be. Titration Curve With Strong Acid And Strong Base.
From mungfali.com
Strong Acid And Base Titration Curve Titration Curve With Strong Acid And Strong Base We'll take hydrochloric acid and sodium hydroxide as typical of a strong. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. titration curves for strong acid v strong base. The reaction of the weak acid, acetic. Naoh (aq) + hcl (aq) → nacl. Titration Curve With Strong Acid And Strong Base.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Curve With Strong Acid And Strong Base in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: for the titration of a strong acid with a strong base, the equivalence point occurs at. Titration Curve With Strong Acid And Strong Base.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Titration Curve With Strong Acid And Strong Base the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Compute sample ph at important stages of a titration. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a. Titration Curve With Strong Acid And Strong Base.
From mavink.com
Strong Acid And Base Titration Curve Titration Curve With Strong Acid And Strong Base Compute sample ph at important stages of a titration. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. The reaction of the weak acid, acetic. As we add strong base to a strong acid,. in. Titration Curve With Strong Acid And Strong Base.
From studylib.net
Titration Curve weak base with strong acid START Titration Curve With Strong Acid And Strong Base Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. We'll take. Titration Curve With Strong Acid And Strong Base.
From mungfali.com
Strong Acid And Base Titration Curve Titration Curve With Strong Acid And Strong Base Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. As we add strong base to a strong acid,. titration curves for strong acid v strong base. Compute sample ph at important stages of a titration. the ph curve diagram below represents the titration of a strong acid with a strong base: We'll. Titration Curve With Strong Acid And Strong Base.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve With Strong Acid And Strong Base titration curves for strong acid v strong base. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Compute sample ph at important stages of a titration. the ph curve diagram. Titration Curve With Strong Acid And Strong Base.
From www.numerade.com
Draw the general titration curve for a strong acid titrated with a Titration Curve With Strong Acid And Strong Base We'll take hydrochloric acid and sodium hydroxide as typical of a strong. titration curves for strong acid v strong base. Compute sample ph at important stages of a titration. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be. Titration Curve With Strong Acid And Strong Base.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Titration Curve With Strong Acid And Strong Base titration curves for strong acid v strong base. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: We'll take hydrochloric acid and sodium hydroxide as typical of a strong. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid. Titration Curve With Strong Acid And Strong Base.
From slidetodoc.com
ACID BASE TITRATION INDICATORS Titration a method of Titration Curve With Strong Acid And Strong Base in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. titration curves for strong acid v strong base. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Compute sample ph at important stages of a titration. The reaction of the weak. Titration Curve With Strong Acid And Strong Base.
From present5.com
AcidBase Titrations Barb Fallon AP Chemistry June 2007 Titration Curve With Strong Acid And Strong Base Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Compute sample ph at important stages of a titration. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of. Titration Curve With Strong Acid And Strong Base.
From saylordotorg.github.io
AcidBase Titrations Titration Curve With Strong Acid And Strong Base the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. As we add strong base to a strong acid,. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: The reaction of the weak acid, acetic. Naoh. Titration Curve With Strong Acid And Strong Base.
From mavink.com
Strong Acid And Base Titration Curve Titration Curve With Strong Acid And Strong Base Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: As we add strong base to a strong acid,. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution.. Titration Curve With Strong Acid And Strong Base.
From www.chegg.com
Solved 1. Figure 1 shows the titration curves of four Titration Curve With Strong Acid And Strong Base The reaction of the weak acid, acetic. the ph curve diagram below represents the titration of a strong acid with a strong base: Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid. Titration Curve With Strong Acid And Strong Base.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve With Strong Acid And Strong Base in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. The reaction of the weak acid, acetic. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: the ph curve diagram below represents the titration of. Titration Curve With Strong Acid And Strong Base.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve With Strong Acid And Strong Base the ph curve diagram below represents the titration of a strong acid with a strong base: We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Compute sample ph at important stages of a titration. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: in a titration. Titration Curve With Strong Acid And Strong Base.
From courses.lumenlearning.com
AcidBase Titrations General Chemistry Titration Curve With Strong Acid And Strong Base Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Compute sample ph at important stages of a titration. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00. Titration Curve With Strong Acid And Strong Base.
From saylordotorg.github.io
AcidBase Titrations Titration Curve With Strong Acid And Strong Base the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. titration curves for strong acid v strong base. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. Let’s consider adding a 0.10 m naoh solution to a 25.0. Titration Curve With Strong Acid And Strong Base.
From mungfali.com
Strong Acid And Base Titration Curve Titration Curve With Strong Acid And Strong Base We'll take hydrochloric acid and sodium hydroxide as typical of a strong. As we add strong base to a strong acid,. The reaction of the weak acid, acetic. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. Let’s consider adding a 0.10 m naoh. Titration Curve With Strong Acid And Strong Base.
From mungfali.com
Weak Acid Vs Strong Base Titration Curve Titration Curve With Strong Acid And Strong Base We'll take hydrochloric acid and sodium hydroxide as typical of a strong. titration curves for strong acid v strong base. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: As we add strong base to a strong acid,. in a titration of a weak acid/ base with a strong. Titration Curve With Strong Acid And Strong Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6776086 Titration Curve With Strong Acid And Strong Base the ph curve diagram below represents the titration of a strong acid with a strong base: The reaction of the weak acid, acetic. Compute sample ph at important stages of a titration. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. in a titration of a weak acid/ base with a strong base/acid, the ph. Titration Curve With Strong Acid And Strong Base.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Curve With Strong Acid And Strong Base Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. the ph curve diagram below represents the. Titration Curve With Strong Acid And Strong Base.
From www.youtube.com
Conductometric Titration & Titration Curves // HSC Chemistry YouTube Titration Curve With Strong Acid And Strong Base Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: the ph curve diagram below represents the titration of a strong acid with a strong base: We'll take hydrochloric acid and sodium hydroxide as typical of. Titration Curve With Strong Acid And Strong Base.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Curve With Strong Acid And Strong Base Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. titration curves for strong acid v strong base. We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: for the titration of a. Titration Curve With Strong Acid And Strong Base.
From saylordotorg.github.io
AcidBase Titrations Titration Curve With Strong Acid And Strong Base As we add strong base to a strong acid,. The reaction of the weak acid, acetic. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: Compute sample ph at important stages of a titration. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. the. Titration Curve With Strong Acid And Strong Base.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve With Strong Acid And Strong Base for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Naoh (aq). Titration Curve With Strong Acid And Strong Base.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Titration Curve With Strong Acid And Strong Base As we add strong base to a strong acid,. Compute sample ph at important stages of a titration. the ph curve diagram below represents the titration of a strong acid with a strong base: titration curves for strong acid v strong base. in a titration of a weak acid/ base with a strong base/acid, the ph changes. Titration Curve With Strong Acid And Strong Base.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Titration Curve With Strong Acid And Strong Base the ph curve diagram below represents the titration of a strong acid with a strong base: Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: Compute sample ph at important stages of a titration. . Titration Curve With Strong Acid And Strong Base.
From mungfali.com
Titration Curve Labeled Titration Curve With Strong Acid And Strong Base We'll take hydrochloric acid and sodium hydroxide as typical of a strong. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. Compute sample ph at important stages of a titration. the ph curve diagram below represents the titration of a strong acid with. Titration Curve With Strong Acid And Strong Base.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Titration Curve With Strong Acid And Strong Base the ph curve diagram below represents the titration of a strong acid with a strong base: Compute sample ph at important stages of a titration. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. Naoh (aq) + hcl (aq) → nacl (aq) +. Titration Curve With Strong Acid And Strong Base.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Curve With Strong Acid And Strong Base Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: We'll take hydrochloric acid and sodium hydroxide as typical of a strong. The reaction of the weak acid, acetic. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the. Titration Curve With Strong Acid And Strong Base.
From mavink.com
Conductometric Titration Graph Titration Curve With Strong Acid And Strong Base Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. The reaction of the weak acid, acetic. titration curves for strong acid v strong base. Compute sample ph at important stages of a titration. Let’s consider adding a 0.10 m naoh solution to a 25.0 ml of a 0.10 m hcl solution: the. Titration Curve With Strong Acid And Strong Base.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID3970559 Titration Curve With Strong Acid And Strong Base We'll take hydrochloric acid and sodium hydroxide as typical of a strong. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) before the. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution.. Titration Curve With Strong Acid And Strong Base.
From schoolbag.info
Titration and Buffers Acids and Bases Training MCAT General Titration Curve With Strong Acid And Strong Base for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. As we add strong base to a strong acid,. The reaction of the weak acid, acetic. titration curves for strong acid v strong base. in. Titration Curve With Strong Acid And Strong Base.