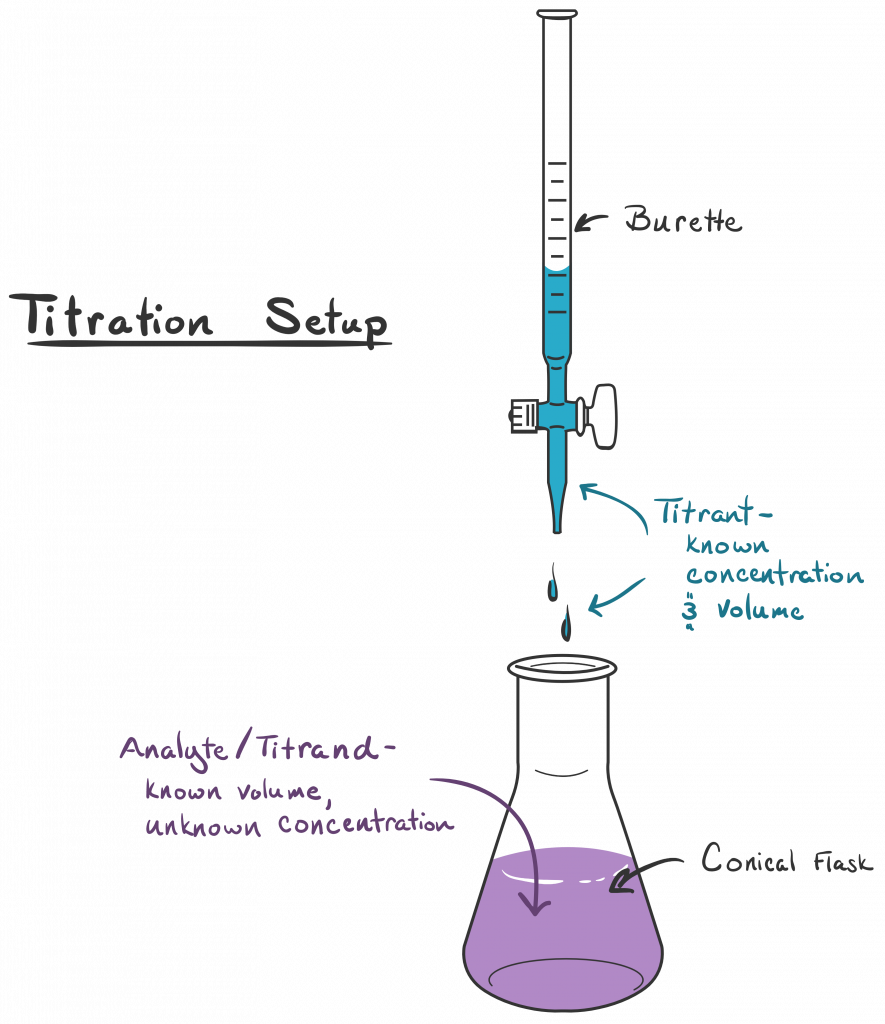
Complexometric Titration In Buffer Solution . For each of the three titrations, therefore, we can easily equate the moles of edta. Use the concept of chemical equilibria in complexometric titrations and calculations. The stoichiometry between edta and each metal ion is 1:1. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric titration. Distinguish among the various types of edta. A complexometric titration uses the formation of a coloured complex to indicate the endpoint. In such a titration, a. Edta, often written as #h_4y# , is a common ligand in complexometric titrations. To do so we need to know the shape of a complexometric titration curve. Alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; This technique is rooted in the. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at.
from cetlkybo.blob.core.windows.net
The stoichiometry between edta and each metal ion is 1:1. After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric titration. To do so we need to know the shape of a complexometric titration curve. Use the concept of chemical equilibria in complexometric titrations and calculations. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. For each of the three titrations, therefore, we can easily equate the moles of edta. This technique is rooted in the. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. In such a titration, a. Alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15;
How To Do Complexometric Titration at Thomas Pegg blog
Complexometric Titration In Buffer Solution Use the concept of chemical equilibria in complexometric titrations and calculations. In such a titration, a. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Use the concept of chemical equilibria in complexometric titrations and calculations. This technique is rooted in the. Distinguish among the various types of edta. A complexometric titration uses the formation of a coloured complex to indicate the endpoint. The stoichiometry between edta and each metal ion is 1:1. Edta, often written as #h_4y# , is a common ligand in complexometric titrations. To do so we need to know the shape of a complexometric titration curve. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. For each of the three titrations, therefore, we can easily equate the moles of edta. Alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric titration.
From www.youtube.com
Complexometric Titration Part2 ‖ EDTA Standardization ‖ Estimation of Complexometric Titration In Buffer Solution Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. Use the concept of chemical equilibria in complexometric titrations and calculations. To do so we need to know the shape of a complexometric titration curve. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents.. Complexometric Titration In Buffer Solution.
From www.studypool.com
SOLUTION Analytical chemistry part 9 complexometric reactions and Complexometric Titration In Buffer Solution For each of the three titrations, therefore, we can easily equate the moles of edta. Edta, often written as #h_4y# , is a common ligand in complexometric titrations. To do so we need to know the shape of a complexometric titration curve. After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric. Complexometric Titration In Buffer Solution.
From www.studypool.com
SOLUTION Complexometric titration note Studypool Complexometric Titration In Buffer Solution The stoichiometry between edta and each metal ion is 1:1. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. Edta, often written as #h_4y# , is a common ligand in complexometric titrations. To do so we need to know the shape of a complexometric titration curve. Use the concept of chemical equilibria in complexometric titrations. Complexometric Titration In Buffer Solution.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts Complexometric Titration In Buffer Solution This technique is rooted in the. A complexometric titration uses the formation of a coloured complex to indicate the endpoint. After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric titration. In such a titration, a. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal. Complexometric Titration In Buffer Solution.
From cetlkybo.blob.core.windows.net
How To Do Complexometric Titration at Thomas Pegg blog Complexometric Titration In Buffer Solution Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. Distinguish among the various types of edta. In such a titration, a. Alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; A complexometric titration uses the formation of a coloured complex to indicate the endpoint. Complexometric titration is a technique in analytical chemistry that. Complexometric Titration In Buffer Solution.
From www.studypool.com
SOLUTION Strength of mgso4 complexometric titration Studypool Complexometric Titration In Buffer Solution Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. To do so we need to know the shape of a complexometric titration curve. A complexometric titration uses the formation of a coloured complex to indicate the endpoint. The stoichiometry between edta and each metal ion is 1:1. Complexometric titration is a technique in analytical chemistry. Complexometric Titration In Buffer Solution.
From www.studypool.com
SOLUTION Analytical chemistry complexometric titration q a Studypool Complexometric Titration In Buffer Solution For each of the three titrations, therefore, we can easily equate the moles of edta. The stoichiometry between edta and each metal ion is 1:1. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. A complexometric titration uses the formation of a coloured complex to indicate the endpoint. Alkaline titration (e.g., ca2+, mg2+) • complex. Complexometric Titration In Buffer Solution.
From www.studypool.com
SOLUTION Complexometric titrations Studypool Complexometric Titration In Buffer Solution Alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; For each of the three titrations, therefore, we can easily equate the moles of edta. This technique is rooted in the. In such a titration, a. To do so we need to know the shape of a complexometric titration curve. Distinguish among the various types of edta. A complexometric. Complexometric Titration In Buffer Solution.
From www.sliderbase.com
Buffers Presentation Chemistry Complexometric Titration In Buffer Solution A complexometric titration uses the formation of a coloured complex to indicate the endpoint. After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric titration. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. This technique is rooted in the.. Complexometric Titration In Buffer Solution.
From www.studypool.com
SOLUTION Volumetric Analysis Titrimetric analysis. Complexometric Complexometric Titration In Buffer Solution This technique is rooted in the. In such a titration, a. The stoichiometry between edta and each metal ion is 1:1. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; A complexometric titration uses the formation of. Complexometric Titration In Buffer Solution.
From collegedunia.com
Complexometric Titration EDTA, Types & Applications Complexometric Titration In Buffer Solution Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. This technique is rooted in the. A complexometric titration uses the formation of a coloured complex to indicate the endpoint. Use the concept of chemical. Complexometric Titration In Buffer Solution.
From facts.net
17 Intriguing Facts About Complexometric Titration Complexometric Titration In Buffer Solution For each of the three titrations, therefore, we can easily equate the moles of edta. In such a titration, a. After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric titration. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. Alkaline titration (e.g., ca2+, mg2+) • complex. Complexometric Titration In Buffer Solution.
From exyyylzwb.blob.core.windows.net
Titration In Pharmaceutical Industry at Jamel Edwards blog Complexometric Titration In Buffer Solution The stoichiometry between edta and each metal ion is 1:1. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Use the concept of chemical equilibria in complexometric titrations and calculations. Edta, often written as #h_4y# , is a common ligand in complexometric titrations. A complexometric titration uses the. Complexometric Titration In Buffer Solution.
From www.studypool.com
SOLUTION Complexometric titration Studypool Complexometric Titration In Buffer Solution Distinguish among the various types of edta. A complexometric titration uses the formation of a coloured complex to indicate the endpoint. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric. Complexometric Titration In Buffer Solution.
From www.studypool.com
SOLUTION Complexometric titrations Studypool Complexometric Titration In Buffer Solution For each of the three titrations, therefore, we can easily equate the moles of edta. This technique is rooted in the. Use the concept of chemical equilibria in complexometric titrations and calculations. After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric titration. In such a titration, a. Complexometric titration is a. Complexometric Titration In Buffer Solution.
From www.youtube.com
COMPLEXOMETRIC TITRATIONS/TITRATIONS/PHARMACEUTICAL ANALYSIS/B PHARM Complexometric Titration In Buffer Solution A complexometric titration uses the formation of a coloured complex to indicate the endpoint. This technique is rooted in the. Edta, often written as #h_4y# , is a common ligand in complexometric titrations. Distinguish among the various types of edta. The stoichiometry between edta and each metal ion is 1:1. Alkaline titration (e.g., ca2+, mg2+) • complex formation constant >. Complexometric Titration In Buffer Solution.
From www.slideshare.net
Complexometric titrations Complexometric Titration In Buffer Solution Distinguish among the various types of edta. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. A complexometric titration uses the formation of a coloured complex to indicate the endpoint. Alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; This technique is rooted in the. The. Complexometric Titration In Buffer Solution.
From www.youtube.com
EXPERIMENT (9) COMPLEXOMETRIC TITRATIONS (TITRATIONS WITH EDTA) YouTube Complexometric Titration In Buffer Solution Edta, often written as #h_4y# , is a common ligand in complexometric titrations. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. In such a titration, a. For each of the three titrations, therefore, we can easily equate the moles of edta. Alkaline titration (e.g., ca2+, mg2+) •. Complexometric Titration In Buffer Solution.
From chemistry.stackexchange.com
analytical chemistry What happens if don't use MgCl₂ in buffer Complexometric Titration In Buffer Solution In such a titration, a. Distinguish among the various types of edta. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric titration. Alkaline titration (e.g., ca2+, mg2+) • complex formation. Complexometric Titration In Buffer Solution.
From www.studypool.com
SOLUTION Complexometric titrations Studypool Complexometric Titration In Buffer Solution In such a titration, a. Alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; For each of the three titrations, therefore, we can easily equate the moles of edta. To do so we need to know the shape of a complexometric titration curve. The stoichiometry between edta and each metal ion is 1:1. Slightly acidic titration (e.g., al3+,. Complexometric Titration In Buffer Solution.
From zanenewswaller.blogspot.com
Why Buffer Solution Is Used in Edta Titration Complexometric Titration In Buffer Solution A complexometric titration uses the formation of a coloured complex to indicate the endpoint. Edta, often written as #h_4y# , is a common ligand in complexometric titrations. Distinguish among the various types of edta. This technique is rooted in the. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. Use the concept of chemical equilibria. Complexometric Titration In Buffer Solution.
From www.metrohm.com
Photometric complexometric titration Metrohm Complexometric Titration In Buffer Solution This technique is rooted in the. In such a titration, a. A complexometric titration uses the formation of a coloured complex to indicate the endpoint. Use the concept of chemical equilibria in complexometric titrations and calculations. The stoichiometry between edta and each metal ion is 1:1. Alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; For each of. Complexometric Titration In Buffer Solution.
From www.youtube.com
Complexometric Titration Using EDTA YouTube Complexometric Titration In Buffer Solution Use the concept of chemical equilibria in complexometric titrations and calculations. After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric titration. Distinguish among the various types of edta. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. In such. Complexometric Titration In Buffer Solution.
From www.slideshare.net
PAI Complexometric titration.(HRB) PPT Complexometric Titration In Buffer Solution After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric titration. In such a titration, a. Distinguish among the various types of edta. Use the concept of chemical equilibria in complexometric titrations and calculations. This technique is rooted in the. A complexometric titration uses the formation of a coloured complex to indicate. Complexometric Titration In Buffer Solution.
From www.studypool.com
SOLUTION Volumetric Analysis Titrimetric analysis. Complexometric Complexometric Titration In Buffer Solution For each of the three titrations, therefore, we can easily equate the moles of edta. In such a titration, a. Alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between. Complexometric Titration In Buffer Solution.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at Complexometric Titration In Buffer Solution Edta, often written as #h_4y# , is a common ligand in complexometric titrations. This technique is rooted in the. For each of the three titrations, therefore, we can easily equate the moles of edta. After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric titration. A complexometric titration uses the formation of. Complexometric Titration In Buffer Solution.
From www.studypool.com
SOLUTION Lecture 7 complexometric titrations Studypool Complexometric Titration In Buffer Solution For each of the three titrations, therefore, we can easily equate the moles of edta. A complexometric titration uses the formation of a coloured complex to indicate the endpoint. Distinguish among the various types of edta. This technique is rooted in the. To do so we need to know the shape of a complexometric titration curve. After the co2+ in. Complexometric Titration In Buffer Solution.
From quizlet.com
Chapter 9 Complexometric Reactions/Titrations Diagram Quizlet Complexometric Titration In Buffer Solution Edta, often written as #h_4y# , is a common ligand in complexometric titrations. For each of the three titrations, therefore, we can easily equate the moles of edta. Use the concept of chemical equilibria in complexometric titrations and calculations. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. Distinguish among the various types of edta.. Complexometric Titration In Buffer Solution.
From www.chemistrylearner.com
Complexometric Titration Definition, Examples, and Applications Complexometric Titration In Buffer Solution In such a titration, a. A complexometric titration uses the formation of a coloured complex to indicate the endpoint. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. The stoichiometry between edta and each. Complexometric Titration In Buffer Solution.
From users.highland.edu
Buffers Complexometric Titration In Buffer Solution After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric titration. A complexometric titration uses the formation of a coloured complex to indicate the endpoint. To do so we need to know the shape of a complexometric titration curve. Distinguish among the various types of edta. For each of the three titrations,. Complexometric Titration In Buffer Solution.
From solutionpharmacy.in
Complexometric Titration Solution Parmacy Complexometric Titration In Buffer Solution The stoichiometry between edta and each metal ion is 1:1. For each of the three titrations, therefore, we can easily equate the moles of edta. In such a titration, a. To do so we need to know the shape of a complexometric titration curve. Distinguish among the various types of edta. Complexometric titration is a technique in analytical chemistry that. Complexometric Titration In Buffer Solution.
From www.youtube.com
Determination of Hardness by Complexometric/ EDTA Method_Water and its Complexometric Titration In Buffer Solution To do so we need to know the shape of a complexometric titration curve. This technique is rooted in the. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Use the concept of chemical equilibria in complexometric titrations and calculations. Edta, often written as #h_4y# , is a. Complexometric Titration In Buffer Solution.
From www.studypool.com
SOLUTION Complexometric titrations microanalysis procedure Studypool Complexometric Titration In Buffer Solution In such a titration, a. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi can even be titrated at. To do so we need to know the shape of a complexometric titration curve. Distinguish among the various types of edta. For each of the three titrations, therefore, we can easily equate the moles of edta. A complexometric titration uses the. Complexometric Titration In Buffer Solution.
From cetlkybo.blob.core.windows.net
How To Do Complexometric Titration at Thomas Pegg blog Complexometric Titration In Buffer Solution Distinguish among the various types of edta. Edta, often written as #h_4y# , is a common ligand in complexometric titrations. The stoichiometry between edta and each metal ion is 1:1. After the co2+ in your unknown is separated from fe3+ it can be quantitatively measured by a complexometric titration. Alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15;. Complexometric Titration In Buffer Solution.
From www.chegg.com
Solved The pH of the Buffer solution used in complexometric Complexometric Titration In Buffer Solution Alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; For each of the three titrations, therefore, we can easily equate the moles of edta. Edta, often written as #h_4y# , is a common ligand in complexometric titrations. A complexometric titration uses the formation of a coloured complex to indicate the endpoint. To do so we need to know. Complexometric Titration In Buffer Solution.