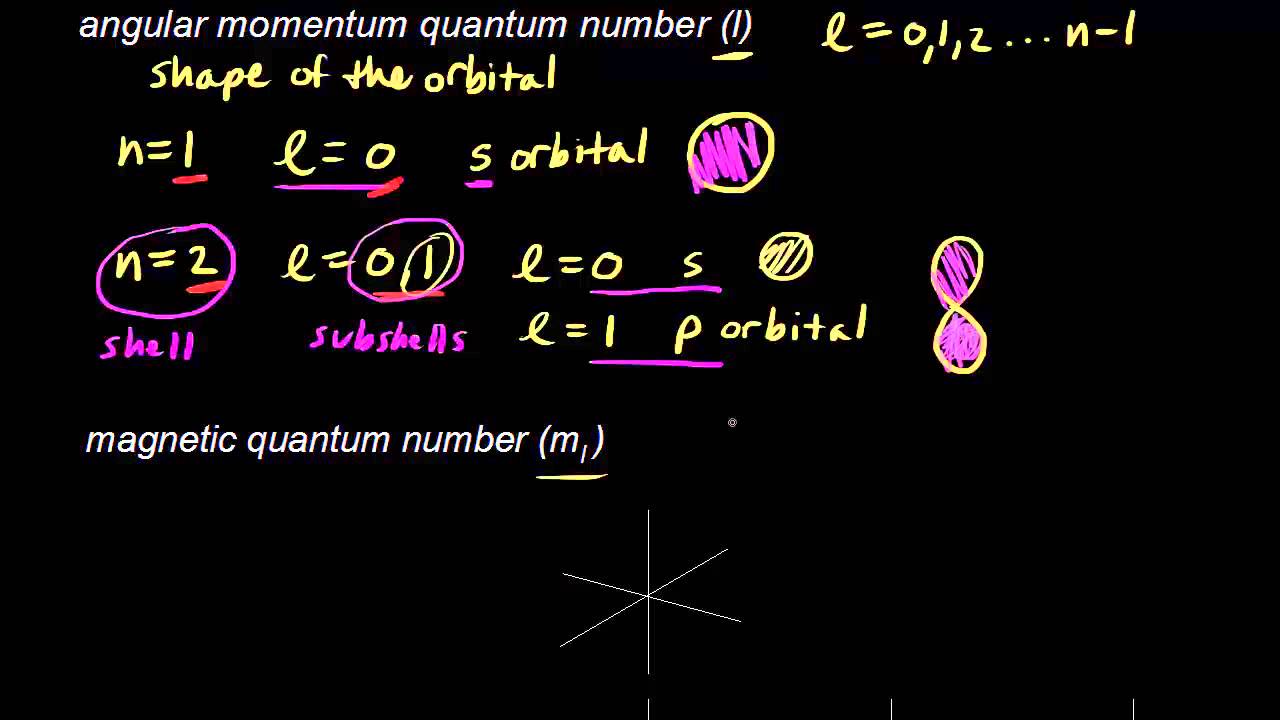
How To Find The Set Of Quantum Numbers . Every electron in an atom has a specific, unique set of these four quantum. The first three quantum numbers. N, l, m l, and m s. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. There are four quantum numbers; For example, potassium is in the fourth period and has a lone electron in its valence shell. An electron in an atom is completely described by four quantum numbers: This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms). The principal quantum number (n), the orbital angular momentum quantum. Electron orbitals, labeled with the value. The quantum numbers are inputs to two key parts of quantum mechanics: Schrodinger’s equation and the pauli exclusion principle. The principal quantum number of an element’s valence electron can be determined from the period in which the element resides in the periodic table. In atoms, there are a total of four quantum numbers: How to find the principal quantum number.
from www.youtube.com
The first three quantum numbers. Schrodinger’s equation and the pauli exclusion principle. An electron in an atom is completely described by four quantum numbers: The quantum numbers are inputs to two key parts of quantum mechanics: For example, potassium is in the fourth period and has a lone electron in its valence shell. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. The principal quantum number (n), the orbital angular momentum quantum. Electron orbitals, labeled with the value. In atoms, there are a total of four quantum numbers: How to find the principal quantum number.
Quantum numbers Electronic structure of atoms Chemistry Khan
How To Find The Set Of Quantum Numbers Their symbols are n, ℓ, mℓand ms. This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms). The principal quantum number of an element’s valence electron can be determined from the period in which the element resides in the periodic table. Their symbols are n, ℓ, mℓand ms. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. There are four quantum numbers; How to find the principal quantum number. In atoms, there are a total of four quantum numbers: For example, potassium is in the fourth period and has a lone electron in its valence shell. N, l, m l, and m s. An electron in an atom is completely described by four quantum numbers: Every electron in an atom has a specific, unique set of these four quantum. Electron orbitals, labeled with the value. The quantum numbers are inputs to two key parts of quantum mechanics: Schrodinger’s equation and the pauli exclusion principle. The principal quantum number (n), the orbital angular momentum quantum.
From www.youtube.com
Quantum Numbers YouTube How To Find The Set Of Quantum Numbers An electron in an atom is completely described by four quantum numbers: In atoms, there are a total of four quantum numbers: How to find the principal quantum number. For example, potassium is in the fourth period and has a lone electron in its valence shell. The principal quantum number (n), the orbital angular momentum quantum. The principal quantum number. How To Find The Set Of Quantum Numbers.
From savekhaoyai.blogspot.com
41 quantum number practice worksheet Worksheet Database How To Find The Set Of Quantum Numbers N, l, m l, and m s. The quantum numbers are inputs to two key parts of quantum mechanics: Every electron in an atom has a specific, unique set of these four quantum. The principal quantum number of an element’s valence electron can be determined from the period in which the element resides in the periodic table. There are four. How To Find The Set Of Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 How To Find The Set Of Quantum Numbers The principal quantum number of an element’s valence electron can be determined from the period in which the element resides in the periodic table. Electron orbitals, labeled with the value. Every electron in an atom has a specific, unique set of these four quantum. This video shows you how to identify or determine the 4 quantum numbers (n, l, ml,. How To Find The Set Of Quantum Numbers.
From www.w3schools.blog
Quantum Numbers W3schools How To Find The Set Of Quantum Numbers N, l, m l, and m s. This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms). The principal quantum number (n), the orbital angular momentum quantum. The first three quantum numbers. The principal quantum number of an element’s valence electron can be determined from the period in which the element resides. How To Find The Set Of Quantum Numbers.
From studylib.net
Quantum Numbers How To Find The Set Of Quantum Numbers The first three quantum numbers. For example, potassium is in the fourth period and has a lone electron in its valence shell. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Electron orbitals, labeled with the value. In atoms, there are a total of four quantum numbers: The principal quantum. How To Find The Set Of Quantum Numbers.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition How To Find The Set Of Quantum Numbers For example, potassium is in the fourth period and has a lone electron in its valence shell. Their symbols are n, ℓ, mℓand ms. Every electron in an atom has a specific, unique set of these four quantum. Schrodinger’s equation and the pauli exclusion principle. This video shows you how to identify or determine the 4 quantum numbers (n, l,. How To Find The Set Of Quantum Numbers.
From www.chegg.com
Solved Write out the values for the set of quantum numbers How To Find The Set Of Quantum Numbers N, l, m l, and m s. Schrodinger’s equation and the pauli exclusion principle. An electron in an atom is completely described by four quantum numbers: Every electron in an atom has a specific, unique set of these four quantum. The principal quantum number (n), the orbital angular momentum quantum. How to find the principal quantum number. In atoms, there. How To Find The Set Of Quantum Numbers.
From www.chegg.com
Solved Which of the following set of quantum numbers How To Find The Set Of Quantum Numbers Their symbols are n, ℓ, mℓand ms. Every electron in an atom has a specific, unique set of these four quantum. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. How to find the principal quantum number. N, l, m l, and m s. For example, potassium is in the. How To Find The Set Of Quantum Numbers.
From www.youtube.com
UPSC Preparation Quantum numbers YouTube How To Find The Set Of Quantum Numbers Every electron in an atom has a specific, unique set of these four quantum. The principal quantum number (n), the orbital angular momentum quantum. The first three quantum numbers. The principal quantum number of an element’s valence electron can be determined from the period in which the element resides in the periodic table. Their symbols are n, ℓ, mℓand ms.. How To Find The Set Of Quantum Numbers.
From www.nagwa.com
Question Video Identifying Which Set of Quantum Numbers Does Not Exist How To Find The Set Of Quantum Numbers Electron orbitals, labeled with the value. Schrodinger’s equation and the pauli exclusion principle. The principal quantum number (n), the orbital angular momentum quantum. In atoms, there are a total of four quantum numbers: This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms). How to find the principal quantum number. The first. How To Find The Set Of Quantum Numbers.
From general.chemistrysteps.com
Quantum Numbers Chemistry Steps How To Find The Set Of Quantum Numbers Every electron in an atom has a specific, unique set of these four quantum. Electron orbitals, labeled with the value. The principal quantum number of an element’s valence electron can be determined from the period in which the element resides in the periodic table. The principal quantum number (n), the orbital angular momentum quantum. An electron in an atom is. How To Find The Set Of Quantum Numbers.
From www.showme.com
How to find elements given quantum numbers ShowMe How To Find The Set Of Quantum Numbers There are four quantum numbers; Their symbols are n, ℓ, mℓand ms. Every electron in an atom has a specific, unique set of these four quantum. How to find the principal quantum number. The quantum numbers are inputs to two key parts of quantum mechanics: The principal quantum number of an element’s valence electron can be determined from the period. How To Find The Set Of Quantum Numbers.
From www.slideserve.com
PPT Chapter 7 Periodicity and Atomic Structure PowerPoint How To Find The Set Of Quantum Numbers Their symbols are n, ℓ, mℓand ms. An electron in an atom is completely described by four quantum numbers: The quantum numbers are inputs to two key parts of quantum mechanics: Every electron in an atom has a specific, unique set of these four quantum. There are four quantum numbers; Schrodinger’s equation and the pauli exclusion principle. N, l, m. How To Find The Set Of Quantum Numbers.
From www.youtube.com
Orbitals, Quantum Numbers & Electron Configuration Multiple Choice How To Find The Set Of Quantum Numbers Electron orbitals, labeled with the value. There are four quantum numbers; For example, potassium is in the fourth period and has a lone electron in its valence shell. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. The principal quantum number of an element’s valence electron can be determined from. How To Find The Set Of Quantum Numbers.
From physicscatalyst.com
Quantum Numbers Chart physicscatalyst's Blog How To Find The Set Of Quantum Numbers Their symbols are n, ℓ, mℓand ms. The quantum numbers are inputs to two key parts of quantum mechanics: The principal quantum number of an element’s valence electron can be determined from the period in which the element resides in the periodic table. The first three quantum numbers. Every electron in an atom has a specific, unique set of these. How To Find The Set Of Quantum Numbers.
From shanimchemistry.blogspot.com
*CHEMISTRY MATRICULATION* QUANTUM NUMBERS How To Find The Set Of Quantum Numbers N, l, m l, and m s. Every electron in an atom has a specific, unique set of these four quantum. This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms). The first three quantum numbers. How to find the principal quantum number. An electron in an atom is completely described by. How To Find The Set Of Quantum Numbers.
From www.slideserve.com
PPT Electron Configurations & Quantum Numbers PowerPoint Presentation How To Find The Set Of Quantum Numbers The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. In atoms, there are a total of four quantum numbers: How to find the principal quantum number. Schrodinger’s equation and the pauli exclusion principle. N, l, m l, and m s. Electron orbitals, labeled with the value. The quantum numbers are. How To Find The Set Of Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID6766216 How To Find The Set Of Quantum Numbers In atoms, there are a total of four quantum numbers: Electron orbitals, labeled with the value. The first three quantum numbers. Their symbols are n, ℓ, mℓand ms. How to find the principal quantum number. The principal quantum number (n), the orbital angular momentum quantum. Every electron in an atom has a specific, unique set of these four quantum. This. How To Find The Set Of Quantum Numbers.
From www.vrogue.co
Quantum Numbers Chart By Emerald City Science Teacher vrogue.co How To Find The Set Of Quantum Numbers An electron in an atom is completely described by four quantum numbers: The principal quantum number of an element’s valence electron can be determined from the period in which the element resides in the periodic table. This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms). Schrodinger’s equation and the pauli exclusion. How To Find The Set Of Quantum Numbers.
From www.toppr.com
Consider the following sets of quantum numbers. (i) n 3, l 0, m 0 How To Find The Set Of Quantum Numbers How to find the principal quantum number. N, l, m l, and m s. The principal quantum number (n), the orbital angular momentum quantum. This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms). The principal quantum number of an element’s valence electron can be determined from the period in which the. How To Find The Set Of Quantum Numbers.
From www.geeksforgeeks.org
Quantum Numbers Principal, Azimuthal, & Spin How To Find The Set Of Quantum Numbers N, l, m l, and m s. The quantum numbers are inputs to two key parts of quantum mechanics: An electron in an atom is completely described by four quantum numbers: The first three quantum numbers. There are four quantum numbers; The principal quantum number of an element’s valence electron can be determined from the period in which the element. How To Find The Set Of Quantum Numbers.
From andreaminsharp.blogspot.com
Set of Quantum Numbers AndreaminSharp How To Find The Set Of Quantum Numbers Their symbols are n, ℓ, mℓand ms. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. The principal quantum number (n), the orbital angular momentum quantum. For example, potassium is in the fourth period and has a lone electron in its valence shell. Schrodinger’s equation and the pauli exclusion principle.. How To Find The Set Of Quantum Numbers.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition How To Find The Set Of Quantum Numbers In atoms, there are a total of four quantum numbers: Their symbols are n, ℓ, mℓand ms. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. How to find the principal quantum number. The first three quantum numbers. There are four quantum numbers; The principal quantum number of an element’s. How To Find The Set Of Quantum Numbers.
From www.youtube.com
Example Writing all sets of 4 Quantum Numbers for the 4d subshell How To Find The Set Of Quantum Numbers Every electron in an atom has a specific, unique set of these four quantum. An electron in an atom is completely described by four quantum numbers: N, l, m l, and m s. For example, potassium is in the fourth period and has a lone electron in its valence shell. Their symbols are n, ℓ, mℓand ms. This video shows. How To Find The Set Of Quantum Numbers.
From www.solutioninn.com
[Solved] Which set of four quantum numbers corresp SolutionInn How To Find The Set Of Quantum Numbers An electron in an atom is completely described by four quantum numbers: The principal quantum number of an element’s valence electron can be determined from the period in which the element resides in the periodic table. Their symbols are n, ℓ, mℓand ms. N, l, m l, and m s. Schrodinger’s equation and the pauli exclusion principle. For example, potassium. How To Find The Set Of Quantum Numbers.
From www.youtube.com
Quantum Number Class 11 Structure of atom Part 4 JEE NEET YouTube How To Find The Set Of Quantum Numbers There are four quantum numbers; How to find the principal quantum number. The quantum numbers are inputs to two key parts of quantum mechanics: An electron in an atom is completely described by four quantum numbers: This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms). The quantum numbers are parameters that. How To Find The Set Of Quantum Numbers.
From study.com
Quantum Numbers on the Periodic Table Definition & Overview Lesson How To Find The Set Of Quantum Numbers The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. The first three quantum numbers. Schrodinger’s equation and the pauli exclusion principle. An electron in an atom is completely described by four quantum numbers: N, l, m l, and m s. Every electron in an atom has a specific, unique set. How To Find The Set Of Quantum Numbers.
From quantum-lesson.blogspot.com
How To Write Set Of Quantum Numbers How To Find The Set Of Quantum Numbers Their symbols are n, ℓ, mℓand ms. Schrodinger’s equation and the pauli exclusion principle. Every electron in an atom has a specific, unique set of these four quantum. Electron orbitals, labeled with the value. N, l, m l, and m s. In atoms, there are a total of four quantum numbers: How to find the principal quantum number. The principal. How To Find The Set Of Quantum Numbers.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin How To Find The Set Of Quantum Numbers The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. The principal quantum number (n), the orbital angular momentum quantum. This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms). The quantum numbers are inputs to two key parts of quantum mechanics: Their. How To Find The Set Of Quantum Numbers.
From mungfali.com
Quantum Numbers For 7th Electron How To Find The Set Of Quantum Numbers The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. The quantum numbers are inputs to two key parts of quantum mechanics: N, l, m l, and m s. The principal quantum number of an element’s valence electron can be determined from the period in which the element resides in the. How To Find The Set Of Quantum Numbers.
From www.youtube.com
Chemistry Lesson 11 Figuring Out the Quantum Numbers YouTube How To Find The Set Of Quantum Numbers N, l, m l, and m s. An electron in an atom is completely described by four quantum numbers: How to find the principal quantum number. There are four quantum numbers; For example, potassium is in the fourth period and has a lone electron in its valence shell. The quantum numbers are inputs to two key parts of quantum mechanics:. How To Find The Set Of Quantum Numbers.
From www.youtube.com
Quantum numbers Electronic structure of atoms Chemistry Khan How To Find The Set Of Quantum Numbers Schrodinger’s equation and the pauli exclusion principle. The first three quantum numbers. Every electron in an atom has a specific, unique set of these four quantum. The principal quantum number (n), the orbital angular momentum quantum. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. There are four quantum numbers;. How To Find The Set Of Quantum Numbers.
From www.priyamstudycentre.com
Quantum Number Orbitals Diagram, Definition, Chart, Shape How To Find The Set Of Quantum Numbers For example, potassium is in the fourth period and has a lone electron in its valence shell. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. In atoms, there are a total of four quantum numbers: Every electron in an atom has a specific, unique set of these four quantum.. How To Find The Set Of Quantum Numbers.
From www.numerade.com
SOLVEDAssign a correct set of four quantum numbers for (a) Each How To Find The Set Of Quantum Numbers In atoms, there are a total of four quantum numbers: This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms). N, l, m l, and m s. Electron orbitals, labeled with the value. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature.. How To Find The Set Of Quantum Numbers.
From general.chemistrysteps.com
Quantum Numbers Chemistry Steps How To Find The Set Of Quantum Numbers For example, potassium is in the fourth period and has a lone electron in its valence shell. Schrodinger’s equation and the pauli exclusion principle. The first three quantum numbers. An electron in an atom is completely described by four quantum numbers: This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms). The. How To Find The Set Of Quantum Numbers.