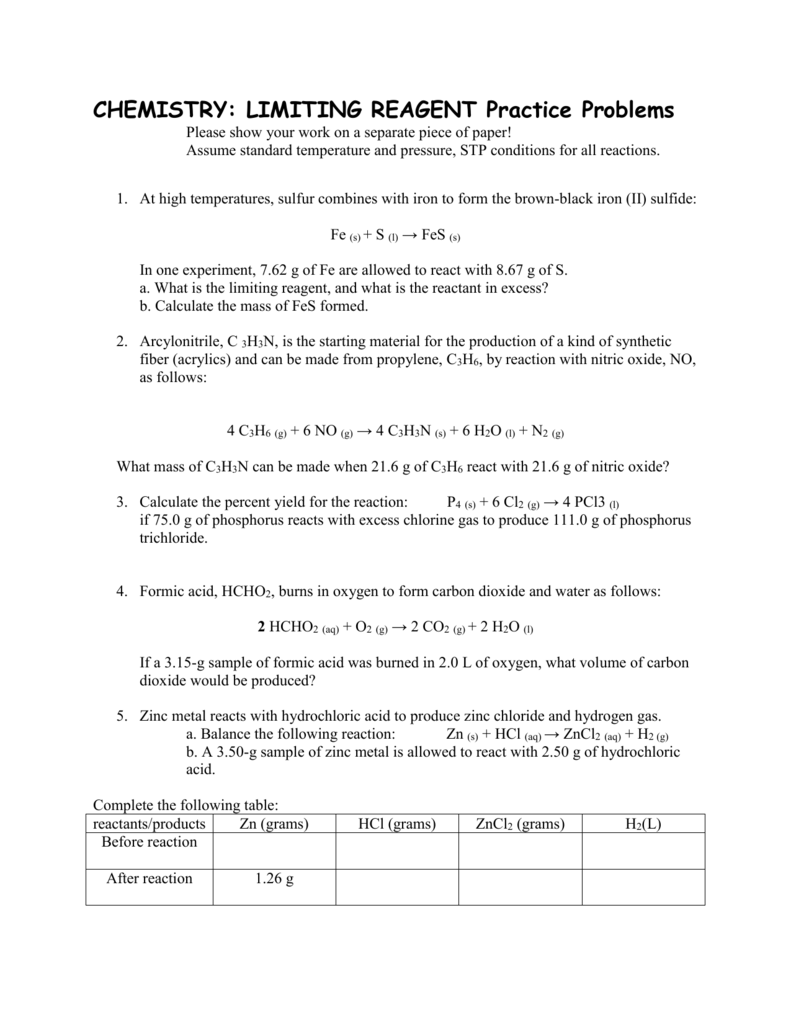
Limiting Reactant Lab Answers . 1) consider the following reaction: Study with quizlet and memorize flashcards containing terms like the limiting reactant is determined in this experiment. The limiting reactant in the salt mixture is k2c2o4⋅h2o. Which reactant is limiting, assuming we started with 30.0 grams. To determine the limiting reactant in a mixture of two soluble salts. To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to Since the limiting reactant will determine the amount of product that can be produced during a reaction, it is important to be able to calculate. Limiting reactant zach, sophie objectives: 2 mass of beaker and salt mixture ( g ) 119 95. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Limiting reactant experiment 8 a. Nh4no3 + na3po4 (nh4)3po4 + nano3. Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker ( g ) 118 94.
from classfullelena.z13.web.core.windows.net
Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker ( g ) 118 94. To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to To determine the limiting reactant in a mixture of two soluble salts. Nh4no3 + na3po4 (nh4)3po4 + nano3. The limiting reactant in the salt mixture is k2c2o4⋅h2o. Study with quizlet and memorize flashcards containing terms like the limiting reactant is determined in this experiment. 1) consider the following reaction: Which reactant is limiting, assuming we started with 30.0 grams. Since the limiting reactant will determine the amount of product that can be produced during a reaction, it is important to be able to calculate. Limiting reactant experiment 8 a.
Limiting Reactant Problems With Answers
Limiting Reactant Lab Answers Since the limiting reactant will determine the amount of product that can be produced during a reaction, it is important to be able to calculate. 1) consider the following reaction: Since the limiting reactant will determine the amount of product that can be produced during a reaction, it is important to be able to calculate. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Study with quizlet and memorize flashcards containing terms like the limiting reactant is determined in this experiment. To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to Which reactant is limiting, assuming we started with 30.0 grams. Limiting reactant zach, sophie objectives: The limiting reactant in the salt mixture is k2c2o4⋅h2o. 2 mass of beaker and salt mixture ( g ) 119 95. Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker ( g ) 118 94. Nh4no3 + na3po4 (nh4)3po4 + nano3. Limiting reactant experiment 8 a. To determine the limiting reactant in a mixture of two soluble salts.
From printablemediajeff.z21.web.core.windows.net
Limiting Reactant Lab Answers Limiting Reactant Lab Answers The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to 1) consider the following reaction: Limiting reactant zach, sophie objectives: Study with quizlet and memorize flashcards containing terms like the limiting. Limiting Reactant Lab Answers.
From www.chegg.com
Solved Limiting Reactant Lab Chem. 101 Limiting Reactant Lab Limiting Reactant Lab Answers 1) consider the following reaction: To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to Nh4no3 + na3po4 (nh4)3po4 + nano3. Limiting reactant zach, sophie objectives: The limiting reactant in the salt mixture is k2c2o4⋅h2o. Which reactant is limiting, assuming we started with 30.0 grams. Precipitation of cac 2 o 4. Limiting Reactant Lab Answers.
From www.scribd.com
Limiting Reagents and Percentage Yield Worksheet answers.doc Zinc Mole (Unit) Limiting Reactant Lab Answers Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker ( g ) 118 94. 1) consider the following reaction: Since the limiting reactant will determine the amount of product that can be produced during a reaction, it is important to be able to calculate. 2. Limiting Reactant Lab Answers.
From lessonmagicwirtz.z13.web.core.windows.net
Limiting Reactants Examples With Answers Limiting Reactant Lab Answers The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Which reactant is limiting, assuming we started with 30.0 grams. Nh4no3 + na3po4 (nh4)3po4 + nano3. Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker. Limiting Reactant Lab Answers.
From ykaahushhush-shutt.blogspot.com
Experiment 8 Pre Laboratory Assignment Limiting Reactant 45+ Pages Analysis in Doc [1.4mb Limiting Reactant Lab Answers Nh4no3 + na3po4 (nh4)3po4 + nano3. The limiting reactant in the salt mixture is k2c2o4⋅h2o. Since the limiting reactant will determine the amount of product that can be produced during a reaction, it is important to be able to calculate. Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2. Limiting Reactant Lab Answers.
From www.studocu.com
Limiting Reactant answers bucher Studocu Limiting Reactant Lab Answers Study with quizlet and memorize flashcards containing terms like the limiting reactant is determined in this experiment. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. To determine the limiting reactant in a mixture of two soluble salts. Which reactant is limiting, assuming we started with 30.0 grams. To. Limiting Reactant Lab Answers.
From lessonlistelois.z13.web.core.windows.net
Limiting Reactant Lab Answers Limiting Reactant Lab Answers Limiting reactant zach, sophie objectives: 1) consider the following reaction: Nh4no3 + na3po4 (nh4)3po4 + nano3. To determine the limiting reactant in a mixture of two soluble salts. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. The limiting reactant in the salt mixture is k2c2o4⋅h2o. Study with quizlet. Limiting Reactant Lab Answers.
From lessonzoneflores88.z13.web.core.windows.net
Limiting Reactant And Percent Yield Worksheets With Answers Limiting Reactant Lab Answers The limiting reactant in the salt mixture is k2c2o4⋅h2o. Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker ( g ) 118 94. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Since the. Limiting Reactant Lab Answers.
From www.studocu.com
HW limiting reactant practice answers 1 Chemistry Name ________________ Limiting Reactant Limiting Reactant Lab Answers Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker ( g ) 118 94. Nh4no3 + na3po4 (nh4)3po4 + nano3. The limiting reactant in the salt mixture is k2c2o4⋅h2o. 1) consider the following reaction: Limiting reactant experiment 8 a. Study with quizlet and memorize flashcards. Limiting Reactant Lab Answers.
From www.studypool.com
SOLUTION Chm 111 lab 5 limiting reagent lab final version Studypool Limiting Reactant Lab Answers Nh4no3 + na3po4 (nh4)3po4 + nano3. To determine the limiting reactant in a mixture of two soluble salts. Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker ( g ) 118 94. The reactant that is consumed first and limits the amount of product(s) that. Limiting Reactant Lab Answers.
From lessonzoneagustin.z21.web.core.windows.net
Limiting Reactant Problems And Answers Pdf Limiting Reactant Lab Answers Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker ( g ) 118 94. To determine the limiting reactant in a mixture of two soluble salts. The limiting reactant in the salt mixture is k2c2o4⋅h2o. Limiting reactant zach, sophie objectives: Which reactant is limiting, assuming. Limiting Reactant Lab Answers.
From www.chegg.com
Experiment 8 Report Sheet Limiting Reactant Dato Lab Limiting Reactant Lab Answers The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Which reactant is limiting, assuming we started with 30.0 grams. Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker ( g ) 118 94. 1). Limiting Reactant Lab Answers.
From www.chegg.com
Limiting Reactant Lab Example Calculations Date Lab Limiting Reactant Lab Answers The limiting reactant in the salt mixture is k2c2o4⋅h2o. 1) consider the following reaction: The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Which reactant is limiting, assuming we started with 30.0 grams. Limiting reactant experiment 8 a. Precipitation of cac 2 o 4 •h 2 o ( g. Limiting Reactant Lab Answers.
From studydbderrico.z13.web.core.windows.net
Chemsheets Limiting Reagents 1 Answers Limiting Reactant Lab Answers 1) consider the following reaction: Since the limiting reactant will determine the amount of product that can be produced during a reaction, it is important to be able to calculate. 2 mass of beaker and salt mixture ( g ) 119 95. To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio. Limiting Reactant Lab Answers.
From learninglibrarybayer.z13.web.core.windows.net
Limiting Reactant Lab Answers Limiting Reactant Lab Answers Study with quizlet and memorize flashcards containing terms like the limiting reactant is determined in this experiment. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Which reactant is limiting, assuming we started with 30.0 grams. Precipitation of cac 2 o 4 •h 2 o ( g ) from. Limiting Reactant Lab Answers.
From learninglibrarybayer.z13.web.core.windows.net
Limiting Reactant Lab Answers Limiting Reactant Lab Answers Limiting reactant zach, sophie objectives: Study with quizlet and memorize flashcards containing terms like the limiting reactant is determined in this experiment. To determine the limiting reactant in a mixture of two soluble salts. Since the limiting reactant will determine the amount of product that can be produced during a reaction, it is important to be able to calculate. Precipitation. Limiting Reactant Lab Answers.
From lessonlistelois.z13.web.core.windows.net
Limiting Reactant Lab Answers Limiting Reactant Lab Answers Which reactant is limiting, assuming we started with 30.0 grams. The limiting reactant in the salt mixture is k2c2o4⋅h2o. Study with quizlet and memorize flashcards containing terms like the limiting reactant is determined in this experiment. Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker. Limiting Reactant Lab Answers.
From www.chegg.com
Solved Experiment 8 Report Sheet Limiting Reactant Desk No. Limiting Reactant Lab Answers Limiting reactant zach, sophie objectives: The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. 2 mass of beaker and salt mixture ( g ) 119 95. Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of. Limiting Reactant Lab Answers.
From worksheetsusanne.z19.web.core.windows.net
Limiting Reactant Problems Step By Step Worksheets Answers Limiting Reactant Lab Answers The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to To determine the limiting reactant in a mixture of two soluble salts. Limiting reactant experiment 8 a. Limiting reactant zach, sophie. Limiting Reactant Lab Answers.
From chemistry.analia-sanchez.net
Limiting Reactant Notes Chemistry Classes / Ronald Reagan S.H.S. Limiting Reactant Lab Answers Limiting reactant experiment 8 a. Limiting reactant zach, sophie objectives: To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to 2 mass of beaker and salt mixture ( g ) 119 95. To determine the limiting reactant in a mixture of two soluble salts. Precipitation of cac 2 o 4 •h. Limiting Reactant Lab Answers.
From books-biblioklept2021.blogspot.com
Experiment 8 Pre Laboratory Assignment Limiting Reactant 60+ Pages Answer [1.1mb] Latest Limiting Reactant Lab Answers To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to Limiting reactant experiment 8 a. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Nh4no3 + na3po4 (nh4)3po4 + nano3. Which reactant is limiting, assuming we started with 30.0 grams.. Limiting Reactant Lab Answers.
From www.chegg.com
Solved Experiment 8 Report Sheet Limiting Reactant Date Lab Limiting Reactant Lab Answers To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. 2 mass of beaker and salt mixture ( g ) 119 95. Limiting reactant experiment 8 a. Which reactant is limiting,. Limiting Reactant Lab Answers.
From lessonlibcohabiters.z13.web.core.windows.net
Answer Key Stoichiometry Worksheet Limiting Reactant Lab Answers The limiting reactant in the salt mixture is k2c2o4⋅h2o. To determine the limiting reactant in a mixture of two soluble salts. 2 mass of beaker and salt mixture ( g ) 119 95. Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker ( g ). Limiting Reactant Lab Answers.
From www.studocu.com
Limiting Reactant (Answers) English Studocu Limiting Reactant Lab Answers To determine the limiting reactant in a mixture of two soluble salts. Limiting reactant experiment 8 a. 1) consider the following reaction: Nh4no3 + na3po4 (nh4)3po4 + nano3. To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to Which reactant is limiting, assuming we started with 30.0 grams. The reactant that. Limiting Reactant Lab Answers.
From printablecampusdrake88.z19.web.core.windows.net
Limiting Reactant Problems Step By Step Worksheets Answers Limiting Reactant Lab Answers To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to Limiting reactant experiment 8 a. Which reactant is limiting, assuming we started with 30.0 grams. Since the limiting reactant will determine the amount of product that can be produced during a reaction, it is important to be able to calculate. The. Limiting Reactant Lab Answers.
From www.onlineworksheet.my.id
Limiting Reactant Worksheet Answers Limiting Reactant Lab Answers To determine the limiting reactant in a mixture of two soluble salts. Nh4no3 + na3po4 (nh4)3po4 + nano3. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Limiting reactant zach, sophie objectives: Limiting reactant experiment 8 a. Since the limiting reactant will determine the amount of product that can. Limiting Reactant Lab Answers.
From learningisidro.z13.web.core.windows.net
Limiting Reactant Lab Answers Limiting Reactant Lab Answers Since the limiting reactant will determine the amount of product that can be produced during a reaction, it is important to be able to calculate. Which reactant is limiting, assuming we started with 30.0 grams. To determine the limiting reactant in a mixture of two soluble salts. Nh4no3 + na3po4 (nh4)3po4 + nano3. Study with quizlet and memorize flashcards containing. Limiting Reactant Lab Answers.
From www.onlineworksheet.my.id
Limiting Reactant Worksheet Answers Limiting Reactant Lab Answers Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker ( g ) 118 94. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. The limiting reactant in the salt mixture is k2c2o4⋅h2o. 1) consider. Limiting Reactant Lab Answers.
From www.studypool.com
SOLUTION Stoichiometry And Limiting Reactant Lab Studypool Limiting Reactant Lab Answers Study with quizlet and memorize flashcards containing terms like the limiting reactant is determined in this experiment. Limiting reactant experiment 8 a. To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to The limiting reactant in the salt mixture is k2c2o4⋅h2o. Precipitation of cac 2 o 4 •h 2 o (. Limiting Reactant Lab Answers.
From classfullelena.z13.web.core.windows.net
Limiting Reactant Problems With Answers Limiting Reactant Lab Answers Precipitation of cac 2 o 4 •h 2 o ( g ) from the salt mixture trial 1 trial 2 1 mass of beaker ( g ) 118 94. To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to Limiting reactant zach, sophie objectives: The limiting reactant in the salt mixture. Limiting Reactant Lab Answers.
From chemistry.analia-sanchez.net
Limiting Reactant Notes Chemistry Classes / Ronald Reagan S.H.S. Limiting Reactant Lab Answers 1) consider the following reaction: Nh4no3 + na3po4 (nh4)3po4 + nano3. 2 mass of beaker and salt mixture ( g ) 119 95. Limiting reactant experiment 8 a. To determine the limiting reactant in a mixture of two soluble salts. Since the limiting reactant will determine the amount of product that can be produced during a reaction, it is important. Limiting Reactant Lab Answers.
From www.vrogue.co
Solved Identify The Limiting Reactant In The Reaction vrogue.co Limiting Reactant Lab Answers Limiting reactant zach, sophie objectives: The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Nh4no3 + na3po4 (nh4)3po4 + nano3. Which reactant is limiting, assuming we started with 30.0 grams. To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to. Limiting Reactant Lab Answers.
From studylib.net
Experiment 4 Limiting Reactant Limiting Reactant Lab Answers To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to Study with quizlet and memorize flashcards containing terms like the limiting reactant is determined in this experiment. 2 mass of beaker and salt mixture ( g ) 119 95. Nh4no3 + na3po4 (nh4)3po4 + nano3. To determine the limiting reactant in. Limiting Reactant Lab Answers.
From www.studocu.com
Zn Cl2 Limiting Reactant Lab Limiting Reactant Lab When a reaction occurs, an equation for the Limiting Reactant Lab Answers The limiting reactant in the salt mixture is k2c2o4⋅h2o. 2 mass of beaker and salt mixture ( g ) 119 95. Study with quizlet and memorize flashcards containing terms like the limiting reactant is determined in this experiment. Since the limiting reactant will determine the amount of product that can be produced during a reaction, it is important to be. Limiting Reactant Lab Answers.
From materialliboster.z19.web.core.windows.net
Limiting Reactant Worksheet Answers Limiting Reactant Lab Answers 1) consider the following reaction: 2 mass of beaker and salt mixture ( g ) 119 95. The reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Nh4no3 + na3po4 (nh4)3po4 + nano3. Since the limiting reactant will determine the amount of product that can be produced during a reaction,. Limiting Reactant Lab Answers.