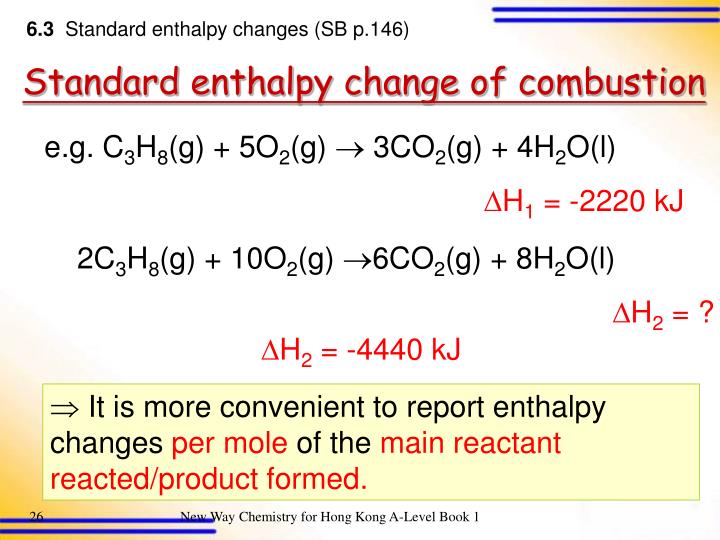
Standard Enthalpy Of Formation H20 Gas . 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. All standard state, 25 °c and 1 bar (written to 1 decimal place). Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),.
from www.slideserve.com
Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under.
PPT Energetics PowerPoint Presentation ID1204656
Standard Enthalpy Of Formation H20 Gas Mallard, eds, nist chemistry webbook, nist standard reference database. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its standard state is zero by definition. All standard state, 25 °c and 1 bar (written to 1 decimal place). Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies of Formation YouTube Standard Enthalpy Of Formation H20 Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),.. Standard Enthalpy Of Formation H20 Gas.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID4097208 Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. Standard Enthalpy Of Formation H20 Gas.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID228912 Standard Enthalpy Of Formation H20 Gas Mallard, eds, nist chemistry webbook, nist standard reference database. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its standard state is zero by definition. The. Standard Enthalpy Of Formation H20 Gas.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Change YouTube Standard Enthalpy Of Formation H20 Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy. Standard Enthalpy Of Formation H20 Gas.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is a measure of the energy released. Standard Enthalpy Of Formation H20 Gas.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For. Standard Enthalpy Of Formation H20 Gas.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas June 2017 Standard Enthalpy Of Formation H20 Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. Mallard, eds, nist chemistry webbook, nist standard reference database. Standard enthalpy change of formation. Standard Enthalpy Of Formation H20 Gas.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation H20 Gas All standard state, 25 °c and 1 bar (written to 1 decimal place). A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard. Standard Enthalpy Of Formation H20 Gas.
From worksheetdbblags.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. A. Standard Enthalpy Of Formation H20 Gas.
From www.slideserve.com
PPT Energetics PowerPoint Presentation ID1204656 Standard Enthalpy Of Formation H20 Gas For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta. Standard Enthalpy Of Formation H20 Gas.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. For example, although oxygen can exist as ozone (o 3),. Standard Enthalpy Of Formation H20 Gas.
From www.chegg.com
Solved The standard enthalpy of formation of H2O(g) at 298 K Standard Enthalpy Of Formation H20 Gas Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. The standard enthalpy of formation of any element in its standard state is zero by definition.. Standard Enthalpy Of Formation H20 Gas.
From www.numerade.com
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 H2O (g) Calculate the Standard Enthalpy Of Formation H20 Gas Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All standard state, 25 °c and 1 bar (written to 1 decimal place). A standard enthalpy. Standard Enthalpy Of Formation H20 Gas.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all answers to three sig figs Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. All standard state,. Standard Enthalpy Of Formation H20 Gas.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation H20 Gas A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is. Standard Enthalpy Of Formation H20 Gas.
From www.toppr.com
Section B Enthalpy of formation & Enthalpy of combustion and Bomb calorimeter The enthalpy of Standard Enthalpy Of Formation H20 Gas For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard. Standard Enthalpy Of Formation H20 Gas.
From www.toppr.com
The standard enthalpy of formation of H2O (l) and Fe2O3 (s) are respectively 286 kJ mol^1 and Standard Enthalpy Of Formation H20 Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy. Standard Enthalpy Of Formation H20 Gas.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation H20 Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Standard enthalpy change of formation (data table) these tables include heat of formation. Standard Enthalpy Of Formation H20 Gas.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. Standard Enthalpy Of Formation H20 Gas.
From www.studypool.com
SOLUTION Standard enthalpy of formation Studypool Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero. Standard Enthalpy Of Formation H20 Gas.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation H20 Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. All standard state, 25 °c and 1 bar (written to 1 decimal place). Standard enthalpy change of formation (data table) these tables include heat of formation. Standard Enthalpy Of Formation H20 Gas.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation H20 Gas A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard. Standard Enthalpy Of Formation H20 Gas.
From www.toppr.com
3. The standard enthalpies of formation of CH4, CO2 and H20 are 74.85,393.5 and 286 kJ/mol Standard Enthalpy Of Formation H20 Gas A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Mallard, eds, nist chemistry webbook, nist standard reference database. Standard enthalpy change of formation (data table) these tables include heat of formation data. Standard Enthalpy Of Formation H20 Gas.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data. Standard Enthalpy Of Formation H20 Gas.
From www.chegg.com
Solved Calculate the standard enthalpy of formation of Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. All standard state, 25 °c and 1 bar (written to 1 decimal place).. Standard Enthalpy Of Formation H20 Gas.
From www.bartleby.com
Answered enthalpy of formation H20 285.83 bartleby Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The standard enthalpy of formation of any element in its standard state. Standard Enthalpy Of Formation H20 Gas.
From www.toppr.com
The standard enthalpy of formation of gaseous H2O at 298 K is 241.82 kJ/mol. Calculate Δ H^o Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly. Standard Enthalpy Of Formation H20 Gas.
From www.bartleby.com
Answered Find the standard enthalpy of formation… bartleby Standard Enthalpy Of Formation H20 Gas A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is a measure of the energy released or consumed. Standard Enthalpy Of Formation H20 Gas.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID6642303 Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Mallard, eds, nist chemistry webbook, nist standard reference database. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in. Standard Enthalpy Of Formation H20 Gas.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation H20 Gas All standard state, 25 °c and 1 bar (written to 1 decimal place). A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example,. Standard Enthalpy Of Formation H20 Gas.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation H20 Gas Mallard, eds, nist chemistry webbook, nist standard reference database. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. All standard state, 25 °c and 1 bar (written to 1 decimal place). Standard enthalpy change of. Standard Enthalpy Of Formation H20 Gas.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation H20 Gas A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation is a measure of the energy released. Standard Enthalpy Of Formation H20 Gas.
From www.bartleby.com
Answered ne standard enthalpy of bartleby Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. All standard state, 25 °c and 1 bar (written to 1 decimal place). Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although. Standard Enthalpy Of Formation H20 Gas.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Heptane Using Standard Standard Enthalpy Of Formation H20 Gas The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. All standard state, 25 °c and 1 bar (written to 1 decimal place). Mallard, eds, nist chemistry webbook, nist standard reference database. Standard enthalpy change of formation (data table) these tables include heat. Standard Enthalpy Of Formation H20 Gas.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation H20 Gas For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation is given the symbol \(\boldsymbol{\delta }_{\boldsymbol{f}}. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table). Standard Enthalpy Of Formation H20 Gas.