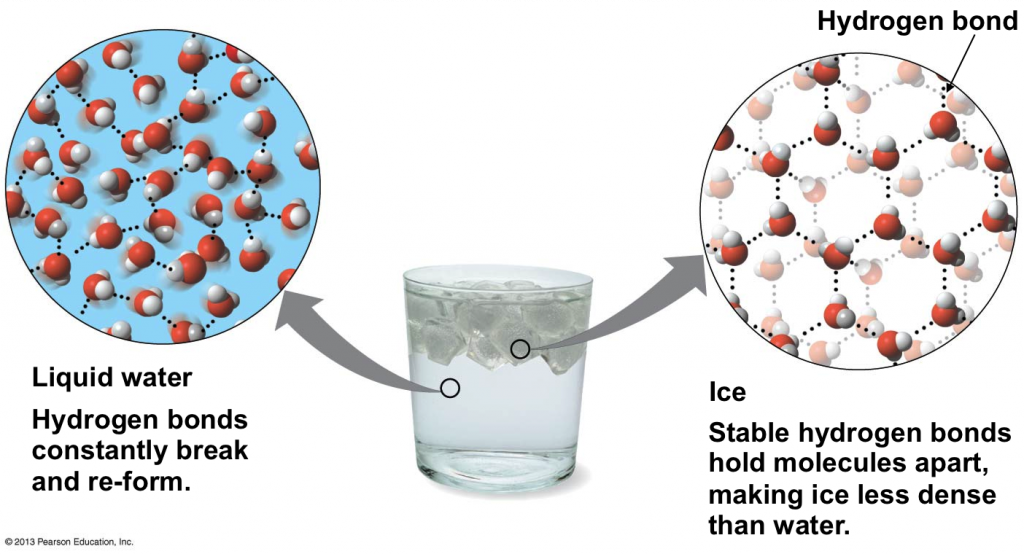
What Happens Entropy When Ice Melts To Liquid Water . Calculate the entropy change for 1.0 mole of ice melting to form. melting of ice occurs in two steps: What is the entropy change of the ice? find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). each degree of motion increases the number of available microstates, resulting in a higher entropy. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. for example, in the case of a melting block of ice, a highly structured and orderly system of water molecules changes into a. the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is called. the enthalpy of fusion for water is 6.01 kj/mol.
from punchlistzero.com
What is the entropy change of the ice? find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). each degree of motion increases the number of available microstates, resulting in a higher entropy. the enthalpy of fusion for water is 6.01 kj/mol. Calculate the entropy change for 1.0 mole of ice melting to form. for example, in the case of a melting block of ice, a highly structured and orderly system of water molecules changes into a. melting of ice occurs in two steps: the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is called. First, the phase change occurs and solid (ice) transforms into liquid water at the melting.
Specific Heat of Ice In Various Units, vs. Water,
What Happens Entropy When Ice Melts To Liquid Water What is the entropy change of the ice? each degree of motion increases the number of available microstates, resulting in a higher entropy. the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is called. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. the enthalpy of fusion for water is 6.01 kj/mol. What is the entropy change of the ice? for example, in the case of a melting block of ice, a highly structured and orderly system of water molecules changes into a. Calculate the entropy change for 1.0 mole of ice melting to form. melting of ice occurs in two steps: find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\).
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, What Happens Entropy When Ice Melts To Liquid Water First, the phase change occurs and solid (ice) transforms into liquid water at the melting. each degree of motion increases the number of available microstates, resulting in a higher entropy. melting of ice occurs in two steps: the heat δq for this process is the energy required to change water from the solid state to the liquid. What Happens Entropy When Ice Melts To Liquid Water.
From mungfali.com
Standard Entropy Chart What Happens Entropy When Ice Melts To Liquid Water Calculate the entropy change for 1.0 mole of ice melting to form. the enthalpy of fusion for water is 6.01 kj/mol. find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). each degree of motion increases the number of available microstates, resulting in a higher entropy.. What Happens Entropy When Ice Melts To Liquid Water.
From www.numerade.com
SOLVED . What is the increase in entropy when one gram of ice at 0° C What Happens Entropy When Ice Melts To Liquid Water the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is called. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. Calculate the entropy change for 1.0 mole of ice melting to form. What is the entropy change of the ice?. What Happens Entropy When Ice Melts To Liquid Water.
From studybrewmaster.z21.web.core.windows.net
What Happens To A Solid When It Melts What Happens Entropy When Ice Melts To Liquid Water melting of ice occurs in two steps: find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). What is the entropy change of the ice? First, the phase change occurs and solid (ice) transforms into liquid water at the melting. Calculate the entropy change for 1.0 mole. What Happens Entropy When Ice Melts To Liquid Water.
From data.allenai.org
changes of state (lesson 0771) TQA explorer What Happens Entropy When Ice Melts To Liquid Water the enthalpy of fusion for water is 6.01 kj/mol. for example, in the case of a melting block of ice, a highly structured and orderly system of water molecules changes into a. each degree of motion increases the number of available microstates, resulting in a higher entropy. What is the entropy change of the ice? First, the. What Happens Entropy When Ice Melts To Liquid Water.
From studylib.net
What Happens to Molecules When a Substance Melts? Ice Liquid What Happens Entropy When Ice Melts To Liquid Water the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is called. Calculate the entropy change for 1.0 mole of ice melting to form. What is the entropy change of the ice? for example, in the case of a melting block of ice, a highly structured. What Happens Entropy When Ice Melts To Liquid Water.
From exojpxgyg.blob.core.windows.net
When Ice Melts What Happens To The Water Molecules at Marjorie Alm blog What Happens Entropy When Ice Melts To Liquid Water First, the phase change occurs and solid (ice) transforms into liquid water at the melting. find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). the enthalpy of fusion for water is 6.01 kj/mol. each degree of motion increases the number of available microstates, resulting in. What Happens Entropy When Ice Melts To Liquid Water.
From huntingwaterfalls.com
What Exactly Happens When an Ice Cube Melts? (Simple Science Explained) What Happens Entropy When Ice Melts To Liquid Water for example, in the case of a melting block of ice, a highly structured and orderly system of water molecules changes into a. Calculate the entropy change for 1.0 mole of ice melting to form. the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is. What Happens Entropy When Ice Melts To Liquid Water.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition What Happens Entropy When Ice Melts To Liquid Water the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is called. melting of ice occurs in two steps: the enthalpy of fusion for water is 6.01 kj/mol. each degree of motion increases the number of available microstates, resulting in a higher entropy. Calculate. What Happens Entropy When Ice Melts To Liquid Water.
From socratic.org
What happens to the energy of its molecules as ice melts into What Happens Entropy When Ice Melts To Liquid Water the enthalpy of fusion for water is 6.01 kj/mol. each degree of motion increases the number of available microstates, resulting in a higher entropy. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. melting of ice occurs in two steps: What is the entropy change of the ice? for example,. What Happens Entropy When Ice Melts To Liquid Water.
From www.alamy.com
Sequence of ice melting into water Stock Photo Alamy What Happens Entropy When Ice Melts To Liquid Water find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). Calculate the entropy change for 1.0 mole of ice melting to form. the enthalpy of fusion for water is 6.01 kj/mol. the heat δq for this process is the energy required to change water from the. What Happens Entropy When Ice Melts To Liquid Water.
From sciencing.com
What Happens to the Temperature of Ice As it Melts? Sciencing What Happens Entropy When Ice Melts To Liquid Water the enthalpy of fusion for water is 6.01 kj/mol. find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). melting of ice occurs in two steps: for example, in the case of a melting block of ice, a highly structured and orderly system of water. What Happens Entropy When Ice Melts To Liquid Water.
From www.snexplores.org
Explainer What are the different states of matter? What Happens Entropy When Ice Melts To Liquid Water find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). First, the phase change occurs and solid (ice) transforms into liquid water at the melting. for example, in the case of a melting block of ice, a highly structured and orderly system of water molecules changes into. What Happens Entropy When Ice Melts To Liquid Water.
From www.youtube.com
When ice melts into water, entropy YouTube What Happens Entropy When Ice Melts To Liquid Water Calculate the entropy change for 1.0 mole of ice melting to form. for example, in the case of a melting block of ice, a highly structured and orderly system of water molecules changes into a. melting of ice occurs in two steps: What is the entropy change of the ice? the enthalpy of fusion for water is. What Happens Entropy When Ice Melts To Liquid Water.
From www.youtube.com
Entropy of Ice Melting YouTube What Happens Entropy When Ice Melts To Liquid Water melting of ice occurs in two steps: each degree of motion increases the number of available microstates, resulting in a higher entropy. What is the entropy change of the ice? the enthalpy of fusion for water is 6.01 kj/mol. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. find the. What Happens Entropy When Ice Melts To Liquid Water.
From www.youtube.com
When Ice Melts, What Happens To The Water Level? 🤔 YouTube What Happens Entropy When Ice Melts To Liquid Water for example, in the case of a melting block of ice, a highly structured and orderly system of water molecules changes into a. each degree of motion increases the number of available microstates, resulting in a higher entropy. the enthalpy of fusion for water is 6.01 kj/mol. What is the entropy change of the ice? melting. What Happens Entropy When Ice Melts To Liquid Water.
From www.youtube.com
Calculating change in Entropy as ice melts on kitchen table P 310 What Happens Entropy When Ice Melts To Liquid Water melting of ice occurs in two steps: for example, in the case of a melting block of ice, a highly structured and orderly system of water molecules changes into a. the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is called. find the. What Happens Entropy When Ice Melts To Liquid Water.
From www.vecteezy.com
Diagram showing how ice melts 433823 Vector Art at Vecteezy What Happens Entropy When Ice Melts To Liquid Water Calculate the entropy change for 1.0 mole of ice melting to form. the enthalpy of fusion for water is 6.01 kj/mol. for example, in the case of a melting block of ice, a highly structured and orderly system of water molecules changes into a. each degree of motion increases the number of available microstates, resulting in a. What Happens Entropy When Ice Melts To Liquid Water.
From thechemistrynotes.com
Entropy Definition, Properties, and Facts What Happens Entropy When Ice Melts To Liquid Water the enthalpy of fusion for water is 6.01 kj/mol. the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is called. for example, in the case of a melting block of ice, a highly structured and orderly system of water molecules changes into a. Calculate. What Happens Entropy When Ice Melts To Liquid Water.
From general.chemistrysteps.com
Entropy and State Change Chemistry Steps What Happens Entropy When Ice Melts To Liquid Water each degree of motion increases the number of available microstates, resulting in a higher entropy. Calculate the entropy change for 1.0 mole of ice melting to form. What is the entropy change of the ice? the enthalpy of fusion for water is 6.01 kj/mol. the heat δq for this process is the energy required to change water. What Happens Entropy When Ice Melts To Liquid Water.
From exojpxgyg.blob.core.windows.net
When Ice Melts What Happens To The Water Molecules at Marjorie Alm blog What Happens Entropy When Ice Melts To Liquid Water each degree of motion increases the number of available microstates, resulting in a higher entropy. What is the entropy change of the ice? melting of ice occurs in two steps: Calculate the entropy change for 1.0 mole of ice melting to form. the heat δq for this process is the energy required to change water from the. What Happens Entropy When Ice Melts To Liquid Water.
From www.pinterest.com
The arrangement of water molecules in solid ice, liquid water and water What Happens Entropy When Ice Melts To Liquid Water First, the phase change occurs and solid (ice) transforms into liquid water at the melting. Calculate the entropy change for 1.0 mole of ice melting to form. the enthalpy of fusion for water is 6.01 kj/mol. What is the entropy change of the ice? each degree of motion increases the number of available microstates, resulting in a higher. What Happens Entropy When Ice Melts To Liquid Water.
From www.slideserve.com
PPT Environmental Cycles of Metabolism PowerPoint Presentation, free What Happens Entropy When Ice Melts To Liquid Water the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is called. What is the entropy change of the ice? each degree of motion increases the number of available microstates, resulting in a higher entropy. for example, in the case of a melting block of. What Happens Entropy When Ice Melts To Liquid Water.
From news.schoolsdo.org
When ice melts on Earth, it happens layer by layer Voxitatis Blog What Happens Entropy When Ice Melts To Liquid Water the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is called. the enthalpy of fusion for water is 6.01 kj/mol. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. each degree of motion increases the number of available. What Happens Entropy When Ice Melts To Liquid Water.
From circuitgonelladrianxm.z22.web.core.windows.net
Show The Phase Change Diagram What Happens Entropy When Ice Melts To Liquid Water melting of ice occurs in two steps: find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). the enthalpy of fusion for water is 6.01 kj/mol. Calculate the entropy change for 1.0 mole of ice melting to form. the heat δq for this process is. What Happens Entropy When Ice Melts To Liquid Water.
From huntingwaterfalls.com
What Exactly Happens When an Ice Cube Melts? (Simple Science Explained) What Happens Entropy When Ice Melts To Liquid Water Calculate the entropy change for 1.0 mole of ice melting to form. each degree of motion increases the number of available microstates, resulting in a higher entropy. melting of ice occurs in two steps: find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). the. What Happens Entropy When Ice Melts To Liquid Water.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk What Happens Entropy When Ice Melts To Liquid Water the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is called. for example, in the case of a melting block of ice, a highly structured and orderly system of water molecules changes into a. First, the phase change occurs and solid (ice) transforms into liquid. What Happens Entropy When Ice Melts To Liquid Water.
From www.youtube.com
What happens to water level, when ice melts। NEET/JEE। Physics।Fluid What Happens Entropy When Ice Melts To Liquid Water find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). What is the entropy change of the ice? the enthalpy of fusion for water is 6.01 kj/mol. the heat δq for this process is the energy required to change water from the solid state to the. What Happens Entropy When Ice Melts To Liquid Water.
From easyscienceforkids.com
Changes in Matter Phases States of Matter Image What Happens Entropy When Ice Melts To Liquid Water find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). First, the phase change occurs and solid (ice) transforms into liquid water at the melting. melting of ice occurs in two steps: What is the entropy change of the ice? the heat δq for this process. What Happens Entropy When Ice Melts To Liquid Water.
From www.slideshare.net
10.3 Entropy and the 2nd law What Happens Entropy When Ice Melts To Liquid Water the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is called. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. each degree of motion increases the number of available microstates, resulting in a higher entropy. find the increase. What Happens Entropy When Ice Melts To Liquid Water.
From www.shmoop.com
Chemistry Phase Changes Shmoop Chemistry What Happens Entropy When Ice Melts To Liquid Water First, the phase change occurs and solid (ice) transforms into liquid water at the melting. Calculate the entropy change for 1.0 mole of ice melting to form. What is the entropy change of the ice? each degree of motion increases the number of available microstates, resulting in a higher entropy. for example, in the case of a melting. What Happens Entropy When Ice Melts To Liquid Water.
From www.dreamstime.com
Entropy Infographic Diagram with Example of Ice Ordered Water What Happens Entropy When Ice Melts To Liquid Water find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). each degree of motion increases the number of available microstates, resulting in a higher entropy. the enthalpy of fusion for water is 6.01 kj/mol. the heat δq for this process is the energy required to. What Happens Entropy When Ice Melts To Liquid Water.
From wirepartnemertines.z5.web.core.windows.net
Changing States Of Matter Diagram What Happens Entropy When Ice Melts To Liquid Water melting of ice occurs in two steps: Calculate the entropy change for 1.0 mole of ice melting to form. each degree of motion increases the number of available microstates, resulting in a higher entropy. find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). for. What Happens Entropy When Ice Melts To Liquid Water.
From www.youtube.com
Calculate the enthalpy change on freezing of 1.0mol of water at 10.0oC What Happens Entropy When Ice Melts To Liquid Water What is the entropy change of the ice? find the increase in entropy of 1.00 kg of ice originally at \(0^oc\), that is melted to form water at \(0^oc\). the heat δq for this process is the energy required to change water from the solid state to the liquid state, and is called. each degree of motion. What Happens Entropy When Ice Melts To Liquid Water.
From physics.stackexchange.com
thermodynamics Why does ice melts, waits for 100 degrees and THEN What Happens Entropy When Ice Melts To Liquid Water each degree of motion increases the number of available microstates, resulting in a higher entropy. What is the entropy change of the ice? Calculate the entropy change for 1.0 mole of ice melting to form. for example, in the case of a melting block of ice, a highly structured and orderly system of water molecules changes into a.. What Happens Entropy When Ice Melts To Liquid Water.