Lead Chromate Plus Water . Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. The ion reacts with water molecules in the solution. Lead(ii) is precipitated by chromate under acidic conditions. Pb 2+ (aq) + cro 4 2− (aq) pbcro 4 (s) [yellow] An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. Lead chromate is highly corrosive and is a strong. A hydrogen ion is lost from one of the ligand water molecules: It is a bright yellow solid that is very poorly soluble. A quick way to identify acids is to see if there is an h (denoting hydrogen) in front of the molecular. Lead chromate is a toxic chemical that can cause serious health problems on inhalation, ingestion or dermal absorption. Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}.
from www.slideshare.net
It is a bright yellow solid that is very poorly soluble. Lead chromate is highly corrosive and is a strong. \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. A hydrogen ion is lost from one of the ligand water molecules: The ion reacts with water molecules in the solution. Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Lead(ii) is precipitated by chromate under acidic conditions. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water.
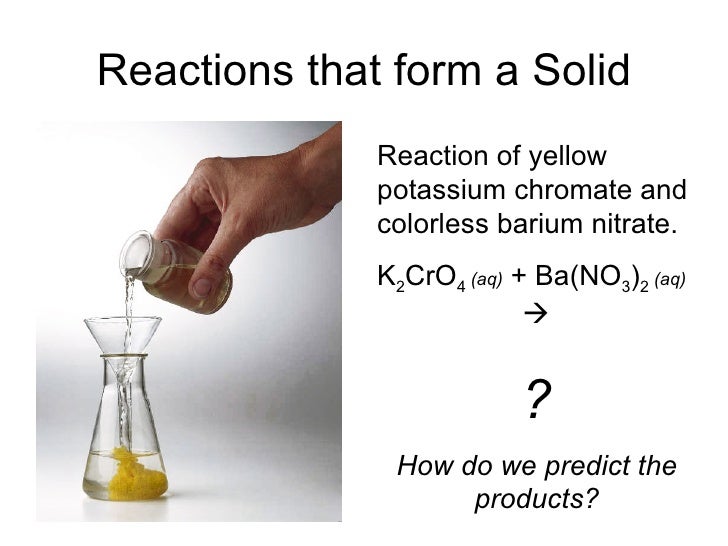
Chapter 8 Reactions in Aqueous Solution
Lead Chromate Plus Water \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. Lead chromate is highly corrosive and is a strong. Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. A quick way to identify acids is to see if there is an h (denoting hydrogen) in front of the molecular. \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. It is a bright yellow solid that is very poorly soluble. Pb 2+ (aq) + cro 4 2− (aq) pbcro 4 (s) [yellow] Lead chromate is a toxic chemical that can cause serious health problems on inhalation, ingestion or dermal absorption. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. A hydrogen ion is lost from one of the ligand water molecules: Lead(ii) is precipitated by chromate under acidic conditions. The ion reacts with water molecules in the solution. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling.
From www.academia.edu
(PDF) Precipitation of lead (II) chromate Folk Narongrit Academia.edu Lead Chromate Plus Water A hydrogen ion is lost from one of the ligand water molecules: The ion reacts with water molecules in the solution. A quick way to identify acids is to see if there is an h (denoting hydrogen) in front of the molecular. Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. Pb 2+ (aq) + cro 4 2−. Lead Chromate Plus Water.
From slideplayer.com
Chemistry ppt download Lead Chromate Plus Water Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. The ion reacts with water molecules in the solution. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. It is a bright yellow solid that is very poorly soluble. Lead chromate is a toxic. Lead Chromate Plus Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Art of Science Lead Chromate Plus Water Lead chromate is a toxic chemical that can cause serious health problems on inhalation, ingestion or dermal absorption. It is a bright yellow solid that is very poorly soluble. A quick way to identify acids is to see if there is an h (denoting hydrogen) in front of the molecular. Lead(ii) is precipitated by chromate under acidic conditions. Lead(ii) chromate. Lead Chromate Plus Water.
From fphoto.photoshelter.com
science chemistry precipitate filtration Fundamental Photographs The Art of Science Lead Chromate Plus Water Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Lead chromate is highly corrosive and is a strong. The ion reacts with water molecules in the solution. A hydrogen ion is lost from one of the ligand water molecules:. Lead Chromate Plus Water.
From www.youtube.com
How to Write the Formula for Lead (II) chromate YouTube Lead Chromate Plus Water Lead(ii) is precipitated by chromate under acidic conditions. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. The ion reacts with water molecules in the solution. A hydrogen ion is lost from one of the ligand water molecules: Pb 2+ (aq) + cro 4 2− (aq) pbcro 4 (s) [yellow] It is a. Lead Chromate Plus Water.
From chem.libretexts.org
Chemistry of Chromium Chemistry LibreTexts Lead Chromate Plus Water Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. A hydrogen ion is lost from one of the ligand water molecules: A quick way to identify acids is to see if there is an h (denoting hydrogen) in. Lead Chromate Plus Water.
From onlinelibrary.wiley.com
Modulating Oxygen Vacancies in Lead Chromate for Photoelectrocatalytic Water Splitting Zhou Lead Chromate Plus Water Pb 2+ (aq) + cro 4 2− (aq) pbcro 4 (s) [yellow] An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. A hydrogen ion is lost from one of. Lead Chromate Plus Water.
From en.wikipedia.org
Chrome yellow Wikipedia Lead Chromate Plus Water It is a bright yellow solid that is very poorly soluble. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. The ion reacts with water molecules in the solution. Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Lead(ii) chromate is an inorganic. Lead Chromate Plus Water.
From fphoto.photoshelter.com
science chemistry precipitate filter Fundamental Photographs The Art of Science Lead Chromate Plus Water Lead(ii) is precipitated by chromate under acidic conditions. Lead chromate is highly corrosive and is a strong. It is a bright yellow solid that is very poorly soluble. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. Lead. Lead Chromate Plus Water.
From www.sciencephoto.com
Lead Chromate Precipitate, 3 of 3 Stock Image C030/8137 Science Photo Library Lead Chromate Plus Water \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. Lead(ii) is precipitated by chromate under acidic conditions. A quick way to identify acids is to see if there is an h (denoting hydrogen) in front of the molecular. Pb 2+ (aq) + cro 4 2− (aq) pbcro 4 (s) [yellow] Lead chromate is a toxic chemical that can cause serious health problems. Lead Chromate Plus Water.
From www.researchgate.net
A Dual‐Ligand Strategy to Regulate the Nucleation and Growth of Lead Chromate Photoanodes for Lead Chromate Plus Water An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Pb 2+ (aq) + cro 4 2− (aq) pbcro 4 (s) [yellow] A. Lead Chromate Plus Water.
From dir.indiamart.com
Lead Chromate at Best Price in India Lead Chromate Plus Water \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Lead(ii) is precipitated by chromate under acidic conditions. It is a bright yellow solid that is very poorly soluble. An acid is a substance that dissociates into hydrogen ions (h +) and anions in. Lead Chromate Plus Water.
From fphoto.photoshelter.com
science chemistry precipitate filtration Fundamental Photographs The Art of Science Lead Chromate Plus Water A hydrogen ion is lost from one of the ligand water molecules: The ion reacts with water molecules in the solution. A quick way to identify acids is to see if there is an h (denoting hydrogen) in front of the molecular. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and. Lead Chromate Plus Water.
From toxicslink.org
Lead Chromate Toxics Link Lead Chromate Plus Water It is a bright yellow solid that is very poorly soluble. \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. Lead chromate is a toxic chemical that can cause serious health problems on inhalation, ingestion or dermal absorption. The ion reacts with water molecules in the solution. Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. Lead chromate is highly. Lead Chromate Plus Water.
From exovbqqgl.blob.core.windows.net
Lead Chromate Solid at Latoya Pardo blog Lead Chromate Plus Water Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Lead chromate is highly corrosive and is a strong. Lead(ii) is precipitated by chromate under acidic conditions. A hydrogen ion is lost from one of the ligand water molecules: A quick way to identify acids is. Lead Chromate Plus Water.
From fphoto.photoshelter.com
science chemistry precipitate filter Fundamental Photographs The Art of Science Lead Chromate Plus Water Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. Lead(ii) is precipitated by chromate under acidic conditions. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes. Lead Chromate Plus Water.
From poliex.me
LEAD CHROMATE PbCrO4 Poliex Lead Chromate Plus Water Pb 2+ (aq) + cro 4 2− (aq) pbcro 4 (s) [yellow] A quick way to identify acids is to see if there is an h (denoting hydrogen) in front of the molecular. Lead chromate is a toxic chemical that can cause serious health problems on inhalation, ingestion or dermal absorption. \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. An acid. Lead Chromate Plus Water.
From fphoto.photoshelter.com
science chemistry precipitate filtration Fundamental Photographs The Art of Science Lead Chromate Plus Water Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. \[\ce{cr(h2o)_6^{3+}. Lead Chromate Plus Water.
From www.reformchem.com
Lead chromate NANTONG REFORM PETROCHEMICAL CO., LTD. Lead Chromate Plus Water Lead chromate is highly corrosive and is a strong. \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. It is a bright yellow solid that is very poorly soluble. Lead(ii) is precipitated by chromate under acidic conditions. An acid is a substance that dissociates into hydrogen ions (h +) and anions. Lead Chromate Plus Water.
From fphoto.photoshelter.com
science chemistry compound lead chromate Fundamental Photographs The Art of Science Lead Chromate Plus Water Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Lead chromate is a toxic chemical that can cause serious health problems on inhalation, ingestion or dermal absorption. \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. The ion reacts with water molecules in the solution. Lead(ii) is. Lead Chromate Plus Water.
From www.sciencephoto.com
Lead chromate precipitate, 1 of 3 Stock Image C036/3125 Science Photo Library Lead Chromate Plus Water Lead(ii) is precipitated by chromate under acidic conditions. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. A hydrogen ion is lost from one of the ligand water molecules: \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+}. Lead Chromate Plus Water.
From www.slideshare.net
Chapter 8 Reactions in Aqueous Solution Lead Chromate Plus Water A hydrogen ion is lost from one of the ligand water molecules: Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Lead chromate is highly corrosive and is a strong. It is a bright yellow solid that is very poorly soluble. The ion reacts with water molecules in the solution.. Lead Chromate Plus Water.
From fphoto.photoshelter.com
science chemistry compound lead chromate Fundamental Photographs The Art of Science Lead Chromate Plus Water It is a bright yellow solid that is very poorly soluble. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals. Lead Chromate Plus Water.
From www.sciencephoto.com
Lead (II) Chloride and Potassium Chromate Stock Image C002/8040 Science Photo Library Lead Chromate Plus Water Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. Lead chromate is highly corrosive and is a strong. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Lead chromate is a toxic. Lead Chromate Plus Water.
From www.alamy.com
PbCrO4 lead chromate CAS 7758976 chemical substance in white plastic laboratory packaging Lead Chromate Plus Water It is a bright yellow solid that is very poorly soluble. Pb 2+ (aq) + cro 4 2− (aq) pbcro 4 (s) [yellow] Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. Lead chromate. Lead Chromate Plus Water.
From cameo.mfa.org
Lead chromate, basic CAMEO Lead Chromate Plus Water Lead chromate is highly corrosive and is a strong. Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water.. Lead Chromate Plus Water.
From www.researchgate.net
Modulating Oxygen Vacancies in Lead Chromate for Photoelectrocatalytic Water Splitting Request PDF Lead Chromate Plus Water It is a bright yellow solid that is very poorly soluble. The ion reacts with water molecules in the solution. Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. Lead(ii) is precipitated by chromate under acidic conditions. Lead chromate is a toxic chemical that can cause serious health problems on inhalation, ingestion or dermal absorption. An acid is. Lead Chromate Plus Water.
From www.chegg.com
Solved Compare the solubility of lead chromate in each of Lead Chromate Plus Water An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. Lead chromate is a toxic chemical that can cause serious health problems on inhalation, ingestion or dermal absorption. Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. Pb 2+ (aq) + cro 4 2− (aq) pbcro 4 (s) [yellow] Two important uses. Lead Chromate Plus Water.
From www.indiamart.com
Lead Chromate Micro Powder at Rs 10/kg Bhankharpur Bhankarpur ID 15946753262 Lead Chromate Plus Water The ion reacts with water molecules in the solution. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. A quick way to identify acids is to see if there is an h (denoting hydrogen) in front of the molecular. Lead chromate is a toxic chemical. Lead Chromate Plus Water.
From www.mosinterchem.com.cn
Lead chromate CAS 7758976 Chemicals supplier from China Lead Chromate Plus Water Lead(ii) is precipitated by chromate under acidic conditions. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. Lead chromate is highly corrosive and is a strong. It is a bright yellow solid that is very poorly soluble. Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium. Lead Chromate Plus Water.
From www.labdepotinc.com
Lead Chromate Lead Chromate Plus Water Pb 2+ (aq) + cro 4 2− (aq) pbcro 4 (s) [yellow] Lead chromate is highly corrosive and is a strong. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. The ion reacts with water molecules in the solution. \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. Two important uses of precipitation reactions are. Lead Chromate Plus Water.
From www.mosinterchem.com.cn
Lead chromate CAS 7758976 Chemicals supplier from China Lead Chromate Plus Water A quick way to identify acids is to see if there is an h (denoting hydrogen) in front of the molecular. Lead chromate is a yellow, orange or red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. Lead(ii) is precipitated by. Lead Chromate Plus Water.
From www.youtube.com
Testing Turmeric Whole adulteration with Lead Chromate FSSAI YouTube Lead Chromate Plus Water It is a bright yellow solid that is very poorly soluble. A hydrogen ion is lost from one of the ligand water molecules: The ion reacts with water molecules in the solution. Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. Lead(ii) is precipitated by chromate under acidic conditions. A quick way to identify acids is to see. Lead Chromate Plus Water.
From www.fishersci.com
Lead Chromate, Powder, ACS, 98, Spectrum Fisher Scientific Lead Chromate Plus Water Lead chromate is a toxic chemical that can cause serious health problems on inhalation, ingestion or dermal absorption. \[\ce{cr(h2o)_6^{3+} + h2o <=> cr(h2o)5(oh)^{2+} + h3o^{+}}. A quick way to identify acids is to see if there is an h (denoting hydrogen) in front of the molecular. A hydrogen ion is lost from one of the ligand water molecules: Two important. Lead Chromate Plus Water.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead chromate Fundamental Photographs The Art of Lead Chromate Plus Water Lead chromate is highly corrosive and is a strong. Lead(ii) chromate is an inorganic compound with the chemical formula pbcro4. An acid is a substance that dissociates into hydrogen ions (h +) and anions in water. A quick way to identify acids is to see if there is an h (denoting hydrogen) in front of the molecular. Lead(ii) is precipitated. Lead Chromate Plus Water.