Fuel Combustion Chemical Equation . On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. Complete and balance chemical equations for combustion reactions. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. A combustion reaction is a reaction in which a substance reacts with. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. A complete combustion is a. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.
from studylib.net
Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Complete and balance chemical equations for combustion reactions. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. A complete combustion is a. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a reaction in which a substance reacts with.
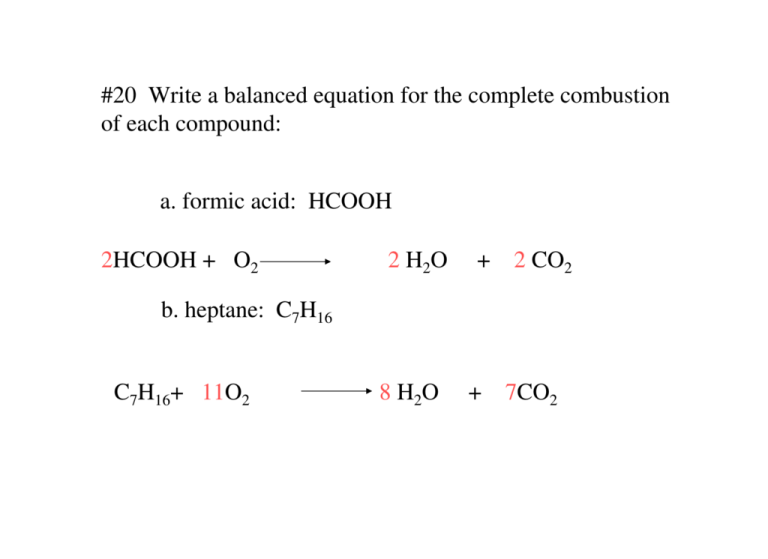
20 Write a balanced equation for the complete combustion of each
Fuel Combustion Chemical Equation Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. Complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A complete combustion is a. A combustion reaction is a reaction in which a substance reacts with. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2.
From www.chegg.com
Solved 2. Combustion Stoichiometry and Properties of a Gas Fuel Combustion Chemical Equation A complete combustion is a. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a reaction. Fuel Combustion Chemical Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Combustion Of Gasoline Tessshebaylo Fuel Combustion Chemical Equation A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. Complete and balance chemical equations for combustion reactions. A combustion reaction. Fuel Combustion Chemical Equation.
From www.youtube.com
Fuels and Combustion Part 6 (Important Combustion equations) YouTube Fuel Combustion Chemical Equation A complete combustion is a. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Complete and balance chemical equations for combustion reactions. On a mole or a volume basis, dry air is. Fuel Combustion Chemical Equation.
From www.thoughtco.com
What Is a Combustion Reaction? Definition and Examples Fuel Combustion Chemical Equation A combustion reaction is a reaction in which a substance reacts with. Complete and balance chemical equations for combustion reactions. A complete combustion is a. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. Combustion involves the oxidation of a fuel, ideally leading,. Fuel Combustion Chemical Equation.
From www.youtube.com
Balancing Combustion Reactions Chemistry Tutorial YouTube Fuel Combustion Chemical Equation A complete combustion is a. A combustion reaction is a reaction in which a substance reacts with. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light. Fuel Combustion Chemical Equation.
From www.slideserve.com
PPT Fuels PowerPoint Presentation, free download ID6911564 Fuel Combustion Chemical Equation A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A complete combustion is a. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. Complete and balance chemical equations for combustion reactions.. Fuel Combustion Chemical Equation.
From www.youtube.com
Balancing equations combustion Reactions meriSTEM YouTube Fuel Combustion Chemical Equation A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a reaction in which a substance reacts with. A complete combustion is a. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the. Fuel Combustion Chemical Equation.
From www.researchgate.net
Set of reactions involved in fuel combustion process in FBR Download Fuel Combustion Chemical Equation Complete and balance chemical equations for combustion reactions. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. A combustion reaction is a reaction in which a substance reacts with. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion involves the. Fuel Combustion Chemical Equation.
From organicvsa.weebly.com
Combustion equations 4 chemical equations and stoichiometry organicvsa Fuel Combustion Chemical Equation A complete combustion is a. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion involves the oxidation of a. Fuel Combustion Chemical Equation.
From www.chemicals.co.uk
Examples of Combustion Reactions in Chemistry The Chemistry Blog Fuel Combustion Chemical Equation A combustion reaction is a reaction in which a substance reacts with. Complete and balance chemical equations for combustion reactions. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. A combustion. Fuel Combustion Chemical Equation.
From www.slideserve.com
PPT Combustion Basics PowerPoint Presentation, free download ID1194415 Fuel Combustion Chemical Equation On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. Complete and balance chemical equations for combustion reactions. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. A complete. Fuel Combustion Chemical Equation.
From www.vrogue.co
Balanced Chemical Equation For The Combustion Of Biod vrogue.co Fuel Combustion Chemical Equation Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. A combustion reaction is a reaction in which a substance. Fuel Combustion Chemical Equation.
From www.numerade.com
⏩SOLVEDThe combustion of gasoline produces carbon dioxide and… Numerade Fuel Combustion Chemical Equation A combustion reaction is a reaction in which a substance reacts with. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. Complete and balance chemical equations for combustion reactions. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such. Fuel Combustion Chemical Equation.
From www.slideserve.com
PPT The combustion process is a chemical reaction whereby fuel is Fuel Combustion Chemical Equation A combustion reaction is a reaction in which a substance reacts with. Complete and balance chemical equations for combustion reactions. A complete combustion is a. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. Stoichiometric or theoretical combustion is the ideal combustion process where fuel. Fuel Combustion Chemical Equation.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Fuel Combustion Chemical Equation On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. Complete and balance chemical equations for combustion reactions. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. A combustion reaction is a reaction in which a substance reacts with. Fuel Combustion Chemical Equation.
From www.shalom-education.com
Fuels and Combustion KS3 Chemistry Revision Fuel Combustion Chemical Equation Complete and balance chemical equations for combustion reactions. A complete combustion is a. A combustion reaction is a reaction in which a substance reacts with. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. Stoichiometric or theoretical combustion is the ideal combustion process. Fuel Combustion Chemical Equation.
From www.slideserve.com
PPT Combustion PowerPoint Presentation, free download ID1992427 Fuel Combustion Chemical Equation Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. A combustion reaction is a reaction in which a substance reacts with. Complete and balance chemical equations for combustion reactions. A complete combustion is a. On a mole or a volume basis, dry air is composed. Fuel Combustion Chemical Equation.
From studylib.net
20 Write a balanced equation for the complete combustion of each Fuel Combustion Chemical Equation Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. A combustion reaction is a reaction in which a substance reacts with. A complete combustion is a. Complete and balance chemical equations for combustion reactions. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation. Fuel Combustion Chemical Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For The Complete Combustion Of Gasoline Fuel Combustion Chemical Equation A combustion reaction is a reaction in which a substance reacts with. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A complete combustion is a. Complete and balance chemical equations for. Fuel Combustion Chemical Equation.
From www.slideserve.com
PPT Heat of Combustion PowerPoint Presentation, free download ID Fuel Combustion Chemical Equation Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. A combustion reaction is a reaction in which a substance reacts with. A complete combustion is a. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. A combustion reaction is a. Fuel Combustion Chemical Equation.
From wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Fuel Combustion Chemical Equation Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. A combustion reaction is a reaction in which a substance reacts with. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. On. Fuel Combustion Chemical Equation.
From www.slideserve.com
PPT Crude Oil PowerPoint Presentation, free download ID1820328 Fuel Combustion Chemical Equation A combustion reaction is a reaction in which a substance reacts with. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. On. Fuel Combustion Chemical Equation.
From signalticket9.pythonanywhere.com
Looking Good Ch4 Combustion A Level Chemistry Ocr Data Sheet Fuel Combustion Chemical Equation On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. A combustion reaction is a reaction in which a substance. Fuel Combustion Chemical Equation.
From preparatorychemistry.com
Combustion Analysis Fuel Combustion Chemical Equation Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. A combustion reaction is a reaction in which a substance. Fuel Combustion Chemical Equation.
From ar.inspiredpencil.com
Gasoline Combustion Reaction Fuel Combustion Chemical Equation Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. A complete combustion is a. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9%. Fuel Combustion Chemical Equation.
From www.youtube.com
Balanced Equation for the Combustion of Methane (CH4) YouTube Fuel Combustion Chemical Equation A combustion reaction is a reaction in which a substance reacts with. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne,. Fuel Combustion Chemical Equation.
From revisechemistry.uk
Carbon Compounds as Fuels and Feedstock AQA C7 revisechemistry.uk Fuel Combustion Chemical Equation Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. A complete combustion is a. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as. Fuel Combustion Chemical Equation.
From www.youtube.com
Writing equations for Combustion Reactions YouTube Fuel Combustion Chemical Equation Complete and balance chemical equations for combustion reactions. A complete combustion is a. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. A combustion reaction is a reaction in. Fuel Combustion Chemical Equation.
From www.youtube.com
Balancing combustion reaction equation Gasoline Chemistry YouTube Fuel Combustion Chemical Equation A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. A complete combustion is a. Complete and balance chemical equations for. Fuel Combustion Chemical Equation.
From www.researchgate.net
(PDF) Combustion of fuels Fuel Combustion Chemical Equation Complete and balance chemical equations for combustion reactions. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. A combustion reaction is a reaction in which a substance reacts with. A complete combustion is a. On a mole or a volume basis, dry air is composed. Fuel Combustion Chemical Equation.
From worksheetsufertatstl.z21.web.core.windows.net
How To Solve Combustion Reaction Fuel Combustion Chemical Equation Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of. Fuel Combustion Chemical Equation.
From ar.inspiredpencil.com
Gasoline Combustion Reaction Fuel Combustion Chemical Equation A complete combustion is a. Complete and balance chemical equations for combustion reactions. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light. Fuel Combustion Chemical Equation.
From www.chemistrylearner.com
Combustion Reaction Definition, Characteristics & Examples Fuel Combustion Chemical Equation Combustion involves the oxidation of a fuel, ideally leading, for an organic fuel such as octane or ethanol, to the formation of carbon dioxide. A combustion reaction is a reaction in which a substance reacts with. A complete combustion is a. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form. Fuel Combustion Chemical Equation.
From sciencenotes.org
Combustion Reaction Definition and Examples Fuel Combustion Chemical Equation A complete combustion is a. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction in which a substance reacts with. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is. Fuel Combustion Chemical Equation.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation ID6818296 Fuel Combustion Chemical Equation Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction in which a substance reacts with. On a mole or a volume basis, dry air is composed of 20.9% o2, 78.1% n2, 0.9% ar, and small amounts of co2, he, ne, h2.. Fuel Combustion Chemical Equation.