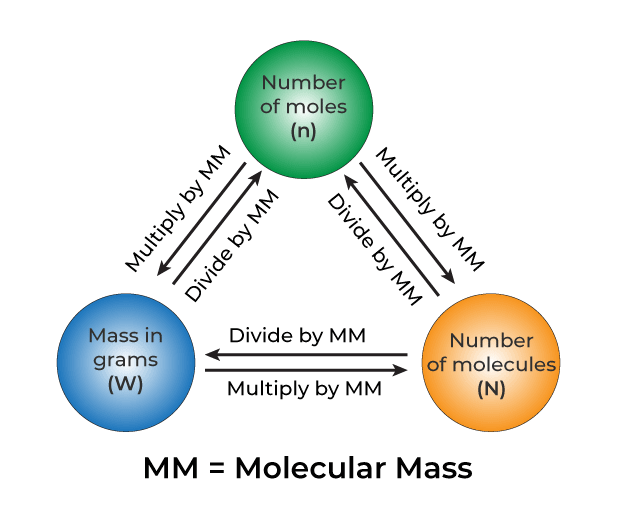
What Is Mole Definition In Chemistry . the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. the mole is the unit of measurement in the international system of units (si) for amount of substance. It is defined as the amount of a. It's one of the seven base units in the international system of units (si). a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. a mole is simply a unit of measurement. In science, this is usually. chemistry uses a unit called mole.
from domdom.ber-engineering.com
mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. a mole is simply a unit of measurement. It's one of the seven base units in the international system of units (si). In science, this is usually. It is defined as the amount of a. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. chemistry uses a unit called mole. the mole is the unit of measurement in the international system of units (si) for amount of substance.
Mole Concept Definition, Formula, Examples, and FAQs
What Is Mole Definition In Chemistry a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. It is defined as the amount of a. In science, this is usually. the mole is the unit of measurement in the international system of units (si) for amount of substance. chemistry uses a unit called mole. a mole is simply a unit of measurement. the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. It's one of the seven base units in the international system of units (si). a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities.
From www.thoughtco.com
What Is a Mole in Chemistry? What Is Mole Definition In Chemistry It is defined as the amount of a. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. a mole is simply a unit of measurement. chemistry uses a unit called mole. In science, this is usually. It's one of the seven base units in the international system of. What Is Mole Definition In Chemistry.
From www.slideserve.com
PPT General Chemistry I Virginia State University PowerPoint What Is Mole Definition In Chemistry In science, this is usually. chemistry uses a unit called mole. the mole is the unit of measurement in the international system of units (si) for amount of substance. It's one of the seven base units in the international system of units (si). the mole is a unit used to measure the number of atoms, molecules, or. What Is Mole Definition In Chemistry.
From www.slideserve.com
PPT The Chemistry MOLE By Mark Silverman PowerPoint Presentation What Is Mole Definition In Chemistry In science, this is usually. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. It's one of the seven base units in the international system of units (si). chemistry uses a unit called mole. a mole is defined as a chemical unit, defined to be 6.022 x 10. What Is Mole Definition In Chemistry.
From www.youtube.com
What is a Chemistry Mole....Explained! YouTube What Is Mole Definition In Chemistry a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. It's one of the seven base units in the international system of units (si).. What Is Mole Definition In Chemistry.
From ar.inspiredpencil.com
Mole Definition Chemistry What Is Mole Definition In Chemistry In science, this is usually. a mole is simply a unit of measurement. It's one of the seven base units in the international system of units (si). the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. chemistry uses a unit. What Is Mole Definition In Chemistry.
From www.pinterest.com
Mole definition The mole is the unit of amount in chemistry. a What Is Mole Definition In Chemistry the mole is the unit of measurement in the international system of units (si) for amount of substance. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. a mole. What Is Mole Definition In Chemistry.
From surfguppy.com
Mole Archives Surfguppy Chemistry made easy visual learning What Is Mole Definition In Chemistry In science, this is usually. a mole is simply a unit of measurement. chemistry uses a unit called mole. the mole is the unit of measurement in the international system of units (si) for amount of substance. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. It's. What Is Mole Definition In Chemistry.
From learningwencelrc.z21.web.core.windows.net
Avogadro's Number And Mole Concept What Is Mole Definition In Chemistry It's one of the seven base units in the international system of units (si). a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. chemistry uses a unit called mole. a mole is simply a unit of measurement. In science, this is usually. mole, in chemistry, a standard. What Is Mole Definition In Chemistry.
From sciencenotes.org
What Is a Mole In Chemistry? Definition What Is Mole Definition In Chemistry In science, this is usually. It's one of the seven base units in the international system of units (si). chemistry uses a unit called mole. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. a mole is simply a unit of measurement. mole, in chemistry, a standard. What Is Mole Definition In Chemistry.
From www.slideserve.com
PPT The Mole PowerPoint Presentation, free download ID5684599 What Is Mole Definition In Chemistry the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. the mole is the unit of measurement in the international system of units. What Is Mole Definition In Chemistry.
From www.slideserve.com
PPT The Mole Concept PowerPoint Presentation, free download ID4787514 What Is Mole Definition In Chemistry In science, this is usually. It's one of the seven base units in the international system of units (si). chemistry uses a unit called mole. a mole is simply a unit of measurement. the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a. What Is Mole Definition In Chemistry.
From www.teachoo.com
Mole Concept Definition, Formulas and Examples Teachoo What Is Mole Definition In Chemistry In science, this is usually. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. It's one of the seven base units in the international system of units (si). a mole is simply a unit of measurement. It is defined as the amount of a. a mole is defined. What Is Mole Definition In Chemistry.
From thechemistrynotes.com
Mole Concept and Molar Mass What Is Mole Definition In Chemistry In science, this is usually. chemistry uses a unit called mole. It is defined as the amount of a. a mole is simply a unit of measurement. the mole is the unit of measurement in the international system of units (si) for amount of substance. mole, in chemistry, a standard scientific unit for measuring large quantities. What Is Mole Definition In Chemistry.
From www.myxxgirl.com
Avogadro S Number And Mole Mole Definition Class Chemistry My XXX Hot What Is Mole Definition In Chemistry It's one of the seven base units in the international system of units (si). the mole is the unit of measurement in the international system of units (si) for amount of substance. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. a mole is simply a unit of. What Is Mole Definition In Chemistry.
From letstalkscience.ca
Moles and Molarity Counting and Chemistry from Donuts to Soap Let's What Is Mole Definition In Chemistry It's one of the seven base units in the international system of units (si). a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass.. What Is Mole Definition In Chemistry.
From www.slideserve.com
PPT THE MOLE CONCEPT PowerPoint Presentation, free download ID442205 What Is Mole Definition In Chemistry In science, this is usually. chemistry uses a unit called mole. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. It is defined as the amount of a. the mole is the unit of measurement in the international system of units (si) for amount of substance. a. What Is Mole Definition In Chemistry.
From www.slideserve.com
PPT The mole PowerPoint Presentation, free download ID1836804 What Is Mole Definition In Chemistry a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula. What Is Mole Definition In Chemistry.
From general.chemistrysteps.com
How To Convert Grams To Moles Chemistry Steps What Is Mole Definition In Chemistry mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. It is defined as the amount of a. In science, this is usually. It's one of the seven base units in the international system of units (si). the mole is the unit of measurement in the international system of units. What Is Mole Definition In Chemistry.
From www.expii.com
Mole Ratios — Definition & Overview Expii What Is Mole Definition In Chemistry the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. In science, this is usually. chemistry uses a unit called mole. It is. What Is Mole Definition In Chemistry.
From www.youtube.com
Chemistry Mole Concept YouTube What Is Mole Definition In Chemistry the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. In science, this is usually. a mole is simply a unit of measurement. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,.. What Is Mole Definition In Chemistry.
From mungfali.com
The Mole Chemistry What Is Mole Definition In Chemistry It is defined as the amount of a. the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. In science, this is usually. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. It's. What Is Mole Definition In Chemistry.
From courses.lumenlearning.com
7.1 The Mole Concept Introductory Chemistry What Is Mole Definition In Chemistry a mole is simply a unit of measurement. the mole is the unit of measurement in the international system of units (si) for amount of substance. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. In science, this is usually. It's one of the seven base units in. What Is Mole Definition In Chemistry.
From definitionvde.blogspot.com
What Is The Definition Of A Mole In Chemistry DEFINITIONVD What Is Mole Definition In Chemistry It's one of the seven base units in the international system of units (si). the mole is the unit of measurement in the international system of units (si) for amount of substance. In science, this is usually. chemistry uses a unit called mole. It is defined as the amount of a. the mole is a unit used. What Is Mole Definition In Chemistry.
From domdom.ber-engineering.com
Mole Concept Definition, Formula, Examples, and FAQs What Is Mole Definition In Chemistry It is defined as the amount of a. a mole is simply a unit of measurement. In science, this is usually. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. the mole is a unit used to measure the number of atoms, molecules, or (in the case of. What Is Mole Definition In Chemistry.
From sciencenotes.org
Mole Ratio Definition and Examples What Is Mole Definition In Chemistry It is defined as the amount of a. In science, this is usually. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. the mole is the unit of measurement in. What Is Mole Definition In Chemistry.
From chemistrymadesimple.net
Why the Mole Concept is the MOST IMPORTANT concept in College Chemistry What Is Mole Definition In Chemistry In science, this is usually. the mole is the unit of measurement in the international system of units (si) for amount of substance. the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. a mole is simply a unit of measurement.. What Is Mole Definition In Chemistry.
From www.vrogue.co
Mole Concept Definition Formula Examples And Faqs 202 vrogue.co What Is Mole Definition In Chemistry chemistry uses a unit called mole. the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. It's one of the seven base units in the international system of units (si). mole, in chemistry, a standard scientific unit for measuring large quantities. What Is Mole Definition In Chemistry.
From ar.inspiredpencil.com
Mole Definition Chemistry What Is Mole Definition In Chemistry a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. mole, in chemistry, a standard scientific unit for measuring large quantities of very. What Is Mole Definition In Chemistry.
From quizlet.com
Chem 08 Reactions & Moles Diagram Quizlet What Is Mole Definition In Chemistry chemistry uses a unit called mole. a mole is simply a unit of measurement. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. In science, this is usually. It. What Is Mole Definition In Chemistry.
From www.youtube.com
What is a Mole and How to Use the Mole in Chemistry YouTube What Is Mole Definition In Chemistry a mole is simply a unit of measurement. In science, this is usually. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. chemistry uses a unit called mole. It's one of the seven base units in the international system of units (si). the mole is the unit. What Is Mole Definition In Chemistry.
From periodictable.me
Introduction to the Mole in Chemistry A Brief Explanation What Is Mole Definition In Chemistry In science, this is usually. the mole is the unit of measurement in the international system of units (si) for amount of substance. the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. a mole is defined as a chemical unit,. What Is Mole Definition In Chemistry.
From ar.inspiredpencil.com
Mole Chemistry What Is Mole Definition In Chemistry the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. a mole is simply a unit of measurement. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. In science, this is usually.. What Is Mole Definition In Chemistry.
From chemistrymysteries.blogspot.com
Chemistry Mysteries Mole Conversions What Is Mole Definition In Chemistry chemistry uses a unit called mole. It's one of the seven base units in the international system of units (si). In science, this is usually. the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass. a mole is simply a unit. What Is Mole Definition In Chemistry.
From iteachly.com
Mole Conversion Worksheet and Activity ⋆ What Is Mole Definition In Chemistry the mole is the unit of measurement in the international system of units (si) for amount of substance. chemistry uses a unit called mole. It is defined as the amount of a. a mole is simply a unit of measurement. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's. What Is Mole Definition In Chemistry.
From mungfali.com
Mole Conversion Flow Chart What Is Mole Definition In Chemistry the mole is the unit of measurement in the international system of units (si) for amount of substance. In science, this is usually. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. a mole is simply a unit of measurement. chemistry uses a unit called mole. It. What Is Mole Definition In Chemistry.