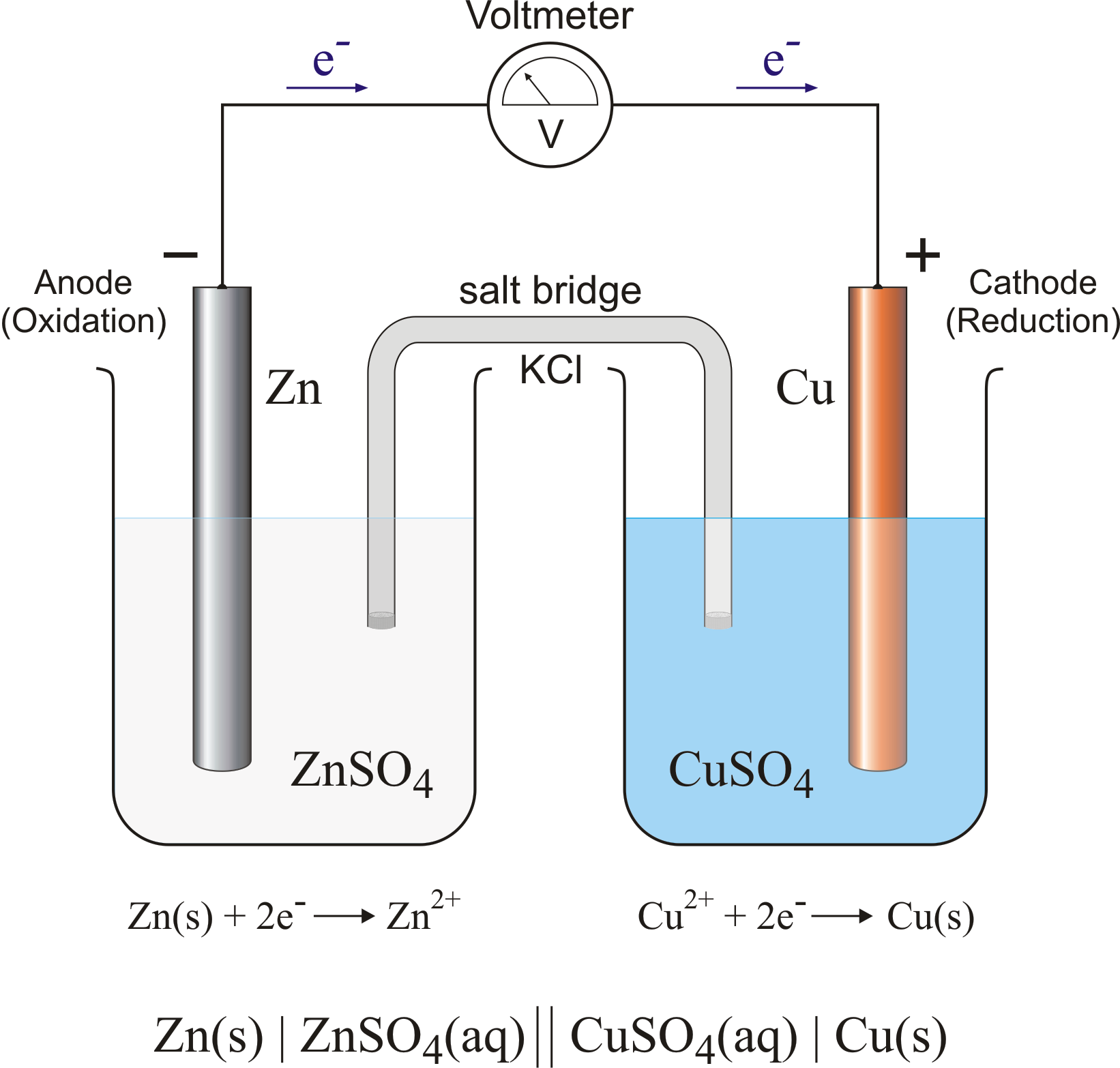
Is A Galvanic Cell Exothermic Or Endothermic . A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. Describe the function of a galvanic cell and its components; I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. One example of a galvanic cell is the common household battery. Use cell notation to symbolize the composition and construction of galvanic cells Sometimes known as a voltaic cell or daniell cell is a galvanic cell. The electrons flow from one chemical reaction to. Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode.
from glossary.periodni.com
One example of a galvanic cell is the common household battery. Describe the function of a galvanic cell and its components; A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. Use cell notation to symbolize the composition and construction of galvanic cells The electrons flow from one chemical reaction to. I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox.
Galvanic cell Chemistry Dictionary & Glossary
Is A Galvanic Cell Exothermic Or Endothermic Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. Describe the function of a galvanic cell and its components; Use cell notation to symbolize the composition and construction of galvanic cells The electrons flow from one chemical reaction to. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. One example of a galvanic cell is the common household battery.
From www.researchgate.net
Schematic depiction of common electrochemical cell configurations Is A Galvanic Cell Exothermic Or Endothermic Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. One example of a galvanic cell is the common household battery. Describe the function of a galvanic cell and its components; Use cell notation to symbolize the composition and construction of galvanic cells I understand the bottom line is that a. Is A Galvanic Cell Exothermic Or Endothermic.
From www.mindomo.com
Metabolism Mind Map Is A Galvanic Cell Exothermic Or Endothermic The electrons flow from one chemical reaction to. I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. Describe the function of a galvanic cell and its components; One example of a galvanic cell is the common. Is A Galvanic Cell Exothermic Or Endothermic.
From www.purechemistry.org
Galvanic cell Is A Galvanic Cell Exothermic Or Endothermic The electrons flow from one chemical reaction to. One example of a galvanic cell is the common household battery. Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. Describe the function of a galvanic cell and its components; A galvanic cell is an electrochemical cell which converts chemical potential energy. Is A Galvanic Cell Exothermic Or Endothermic.
From courses.lumenlearning.com
Galvanic Cells Chemistry Atoms First Is A Galvanic Cell Exothermic Or Endothermic One example of a galvanic cell is the common household battery. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. Describe the function of a galvanic cell and its components; A galvanic cell is an electrochemical cell. Is A Galvanic Cell Exothermic Or Endothermic.
From 7esl.com
Exothermic vs. Endothermic The Main Difference • 7ESL Is A Galvanic Cell Exothermic Or Endothermic I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. Describe the function of a galvanic cell and its components; Sometimes known as a voltaic cell or daniell cell. Is A Galvanic Cell Exothermic Or Endothermic.
From glossary.periodni.com
Galvanic cell Chemistry Dictionary & Glossary Is A Galvanic Cell Exothermic Or Endothermic Describe the function of a galvanic cell and its components; One example of a galvanic cell is the common household battery. Use cell notation to symbolize the composition and construction of galvanic cells I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. The electrons flow from one chemical reaction. Is A Galvanic Cell Exothermic Or Endothermic.
From biochemreview.weebly.com
Galvanic cell BIOChemReview Is A Galvanic Cell Exothermic Or Endothermic The electrons flow from one chemical reaction to. I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. Describe the function of a galvanic cell and its components; One example of a galvanic cell is the common household battery. Cell notation uses the simplest form of each of the equations,. Is A Galvanic Cell Exothermic Or Endothermic.
From www.pinterest.com
Galvanic Cell Animation Galvanic cell, Cell, Electrical energy Is A Galvanic Cell Exothermic Or Endothermic Sometimes known as a voltaic cell or daniell cell is a galvanic cell. I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. One example of a galvanic cell is the common household battery. Cell notation uses the simplest form of each of the equations, and starts with the reaction. Is A Galvanic Cell Exothermic Or Endothermic.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Is A Galvanic Cell Exothermic Or Endothermic Describe the function of a galvanic cell and its components; I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. One example of a galvanic cell is the common household battery. Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode.. Is A Galvanic Cell Exothermic Or Endothermic.
From 5differencebetween.com
5 Difference Between Endothermic and Exothermic Reaction Endothermic Is A Galvanic Cell Exothermic Or Endothermic I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. Describe the function of a galvanic cell and its components; Sometimes known as a voltaic cell or daniell cell is a galvanic cell. Use cell notation to symbolize the composition and construction of galvanic cells A galvanic cell is an. Is A Galvanic Cell Exothermic Or Endothermic.
From edurev.in
Daniel Cell Or Galvanic Cell Class 12 Notes EduRev Is A Galvanic Cell Exothermic Or Endothermic One example of a galvanic cell is the common household battery. Describe the function of a galvanic cell and its components; A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a. Is A Galvanic Cell Exothermic Or Endothermic.
From ar.inspiredpencil.com
Galvanic Cell Labeled Is A Galvanic Cell Exothermic Or Endothermic Sometimes known as a voltaic cell or daniell cell is a galvanic cell. The electrons flow from one chemical reaction to. A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. Cell notation uses the simplest form of each of the equations, and starts with the reaction at the. Is A Galvanic Cell Exothermic Or Endothermic.
From stock.adobe.com
Endothermic Vs Exothermic Reactions Infographic Diagram showing a Is A Galvanic Cell Exothermic Or Endothermic A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. The electrons flow from one chemical reaction to. Use cell notation to symbolize the composition and construction of galvanic cells Sometimes known as a voltaic cell or daniell cell is a galvanic cell. Cell notation uses the simplest form. Is A Galvanic Cell Exothermic Or Endothermic.
From mavink.com
Galvanic Cell Labeled Is A Galvanic Cell Exothermic Or Endothermic A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. Use cell notation to symbolize the composition and construction of galvanic cells I understand the bottom line is that a simple cell converts chemical energy to. Is A Galvanic Cell Exothermic Or Endothermic.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Is A Galvanic Cell Exothermic Or Endothermic Sometimes known as a voltaic cell or daniell cell is a galvanic cell. Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. One example of a galvanic cell is the common household battery. Describe the function of a galvanic cell and its components; I understand the bottom line is that. Is A Galvanic Cell Exothermic Or Endothermic.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902 Is A Galvanic Cell Exothermic Or Endothermic Sometimes known as a voltaic cell or daniell cell is a galvanic cell. A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. The electrons flow from one chemical. Is A Galvanic Cell Exothermic Or Endothermic.
From www.vrogue.co
Galvanic Cell Salt Bridge vrogue.co Is A Galvanic Cell Exothermic Or Endothermic Use cell notation to symbolize the composition and construction of galvanic cells One example of a galvanic cell is the common household battery. Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. Describe the function of a galvanic cell and its components; Sometimes known as a voltaic cell or daniell. Is A Galvanic Cell Exothermic Or Endothermic.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC Is A Galvanic Cell Exothermic Or Endothermic Sometimes known as a voltaic cell or daniell cell is a galvanic cell. A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. The electrons flow from one chemical reaction to. One example of a galvanic cell is the common household battery. Cell notation uses the simplest form of. Is A Galvanic Cell Exothermic Or Endothermic.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Is A Galvanic Cell Exothermic Or Endothermic Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. The electrons flow from one chemical reaction to. I understand the bottom line is that a simple cell converts chemical. Is A Galvanic Cell Exothermic Or Endothermic.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts Is A Galvanic Cell Exothermic Or Endothermic A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. One example of a galvanic cell is the common household battery. Sometimes known as a voltaic cell or daniell cell. Is A Galvanic Cell Exothermic Or Endothermic.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Is A Galvanic Cell Exothermic Or Endothermic One example of a galvanic cell is the common household battery. Use cell notation to symbolize the composition and construction of galvanic cells I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. The electrons flow from one chemical reaction to. Sometimes known as a voltaic cell or daniell cell. Is A Galvanic Cell Exothermic Or Endothermic.
From mungfali.com
Galvanic Cell Diagram Is A Galvanic Cell Exothermic Or Endothermic A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. The electrons flow from one chemical reaction to. Cell notation uses the simplest form of each of the equations, and starts with the reaction at the. Is A Galvanic Cell Exothermic Or Endothermic.
From www.collegesearch.in
Galvanic Cells Definitions, Examples, How They are Created, Principles Is A Galvanic Cell Exothermic Or Endothermic Describe the function of a galvanic cell and its components; One example of a galvanic cell is the common household battery. Use cell notation to symbolize the composition and construction of galvanic cells A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. I understand the bottom line is. Is A Galvanic Cell Exothermic Or Endothermic.
From www.numerade.com
SOLVED [3] (a) Differentiate the exothermic reactions from endothermic Is A Galvanic Cell Exothermic Or Endothermic Sometimes known as a voltaic cell or daniell cell is a galvanic cell. A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. One example of a galvanic cell is the common household battery. The electrons flow from one chemical reaction to. Describe the function of a galvanic cell. Is A Galvanic Cell Exothermic Or Endothermic.
From thechemistrynotes.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle Is A Galvanic Cell Exothermic Or Endothermic I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. One example of a galvanic cell is the common household battery. Use cell notation to symbolize the composition and. Is A Galvanic Cell Exothermic Or Endothermic.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Is A Galvanic Cell Exothermic Or Endothermic Sometimes known as a voltaic cell or daniell cell is a galvanic cell. Describe the function of a galvanic cell and its components; The electrons flow from one chemical reaction to. One example of a galvanic cell is the common household battery. I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a. Is A Galvanic Cell Exothermic Or Endothermic.
From www.youtube.com
Galvanic Cells Explained // Preliminary HSC Chemistry YouTube Is A Galvanic Cell Exothermic Or Endothermic Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. Use cell notation to symbolize the composition and construction of galvanic cells Sometimes known as a voltaic cell or daniell cell is a galvanic cell. Describe the function of a galvanic cell and its components; One example of a galvanic cell. Is A Galvanic Cell Exothermic Or Endothermic.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle Is A Galvanic Cell Exothermic Or Endothermic Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. The electrons flow from one chemical reaction to. One example of a galvanic cell is the common household battery. A galvanic cell is an electrochemical cell which converts. Is A Galvanic Cell Exothermic Or Endothermic.
From electrochemistryh5t34.blogspot.com
We Love Electrochemistry Galvanic Cell Is A Galvanic Cell Exothermic Or Endothermic One example of a galvanic cell is the common household battery. I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. Use cell notation to symbolize the composition and construction of galvanic cells Cell notation uses the. Is A Galvanic Cell Exothermic Or Endothermic.
From www.chemistrylearner.com
Satyam Bhuyan, Author at Chemistry Learner Is A Galvanic Cell Exothermic Or Endothermic Sometimes known as a voltaic cell or daniell cell is a galvanic cell. The electrons flow from one chemical reaction to. One example of a galvanic cell is the common household battery. Use cell notation to symbolize the composition and construction of galvanic cells A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy. Is A Galvanic Cell Exothermic Or Endothermic.
From stock.adobe.com
Vettoriale Stock Voltaic galvanic cell or daniell cell.Redox reaction Is A Galvanic Cell Exothermic Or Endothermic A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a spontaneous chemical reaction. I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. The electrons flow from one chemical reaction to. One example of a galvanic cell is the common household battery.. Is A Galvanic Cell Exothermic Or Endothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Is A Galvanic Cell Exothermic Or Endothermic Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. Describe the function of a galvanic cell and its components; Sometimes known as a voltaic cell or daniell cell is a galvanic cell. A galvanic cell is an electrochemical cell which converts chemical potential energy to electrical potential energy through a. Is A Galvanic Cell Exothermic Or Endothermic.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Is A Galvanic Cell Exothermic Or Endothermic Use cell notation to symbolize the composition and construction of galvanic cells Describe the function of a galvanic cell and its components; I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. The electrons flow from one. Is A Galvanic Cell Exothermic Or Endothermic.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Is A Galvanic Cell Exothermic Or Endothermic The electrons flow from one chemical reaction to. I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. One example of a galvanic cell is the common household battery. Describe the function of a galvanic cell and its components; Cell notation uses the simplest form of each of the equations,. Is A Galvanic Cell Exothermic Or Endothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Is A Galvanic Cell Exothermic Or Endothermic Describe the function of a galvanic cell and its components; One example of a galvanic cell is the common household battery. I understand the bottom line is that a simple cell converts chemical energy to electrical energy in a spontaneous redox. Use cell notation to symbolize the composition and construction of galvanic cells Cell notation uses the simplest form of. Is A Galvanic Cell Exothermic Or Endothermic.