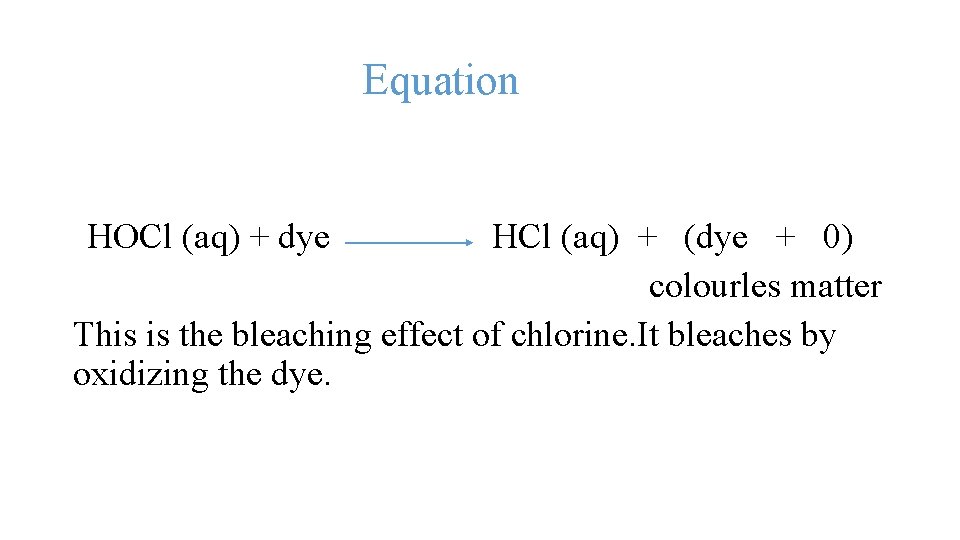
Chlorine With Water Equation . Define disproportionation & describe the role of chlorine in water purification. Chloric (i) acid (hclo) sterilises water by killing bacteria. Use our revision notes to revise the reaction of chlorine with naoh. The following equation presents the hydrolysis reaction (usepa 1999; Chlorine is only slightly soluble in water, with its maximum solubility. Reaction of chlorine with water. When chlorine reacts with water, it forms a mixture of hydrochloric acid, hcl, and chloric acid, hclo. Usually, reactions of chlorine with water are for disinfection purposes. The chlorine mixed with water will produce hypochlorous acid (hocl). Chlorine is available in three forms: Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). The equation for this reaction is: When chlorine gas is dissolved in water, the reaction forms. The different products have some practical uses. Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −.
from slidetodoc.com
Chlorine is only slightly soluble in water, with its maximum solubility. Chlorine reacts with water in different ways depending on the conditions. The reaction of chlorine in water is a disproportionation reaction in which the chlorine gets both oxidised and reduced. Usually, reactions of chlorine with water are for disinfection purposes. Chloric (i) acid (hclo) sterilises water by killing bacteria. Hclo (aq) → h+ (aq) +. The chlorine mixed with water will produce hypochlorous acid (hocl). When chlorine gas is dissolved in water, the reaction forms. The equation for this reaction is: Cl 2 (g) + h 2 o (l).
REACTION OF CHLORINE WITH WATER Chlorine dissolves in
Chlorine With Water Equation Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −. When chlorine gas is dissolved in water, the reaction forms. When chlorine reacts with water, it forms a mixture of hydrochloric acid, hcl, and chloric acid, hclo. The different products have some practical uses. Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −. Usually, reactions of chlorine with water are for disinfection purposes. Chlorine is only slightly soluble in water, with its maximum solubility. Reaction of chlorine with water. Use our revision notes to revise the reaction of chlorine with naoh. Define disproportionation & describe the role of chlorine in water purification. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). Chloric (i) acid (hclo) sterilises water by killing bacteria. Chlorine is available in three forms: The equation for this reaction is: Cl 2 (g) + h 2 o (l). The following equation presents the hydrolysis reaction (usepa 1999;
From openoregon.pressbooks.pub
4.3 AcidBase Reactions Introduction to Chemistry Chlorine With Water Equation When chlorine reacts with water, it forms a mixture of hydrochloric acid, hcl, and chloric acid, hclo. Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −. Define disproportionation & describe the role of chlorine in water purification. The reaction of chlorine in water is a disproportionation reaction in which the chlorine gets both oxidised. Chlorine With Water Equation.
From www.youtube.com
13.1.2 Describe the reactions of chlorine and the chlorides referred to in 13.1.1 with water Chlorine With Water Equation When chlorine reacts with water, it forms a mixture of hydrochloric acid, hcl, and chloric acid, hclo. Define disproportionation & describe the role of chlorine in water purification. The following equation presents the hydrolysis reaction (usepa 1999; Chlorine reacts with water in different ways depending on the conditions. Chloric (i) acid (hclo) sterilises water by killing bacteria. The reaction of. Chlorine With Water Equation.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Hydrogen Chloride In Water Equation Chlorine With Water Equation When chlorine reacts with water, it forms a mixture of hydrochloric acid, hcl, and chloric acid, hclo. The following equation presents the hydrolysis reaction (usepa 1999; Chlorine reacts with water in different ways depending on the conditions. Reaction of chlorine with water. Chloric (i) acid (hclo) sterilises water by killing bacteria. Use our revision notes to revise the reaction of. Chlorine With Water Equation.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Reaction Of Hydrogen Chloride Gas With Water Chlorine With Water Equation The following equation presents the hydrolysis reaction (usepa 1999; Use our revision notes to revise the reaction of chlorine with naoh. Chlorine is available in three forms: When chlorine gas is dissolved in water, the reaction forms. Reaction of chlorine with water. Define disproportionation & describe the role of chlorine in water purification. The different products have some practical uses.. Chlorine With Water Equation.
From www.youtube.com
Chlorine and Water AS Chemistry YouTube Chlorine With Water Equation Cl 2 (g) + h 2 o (l). Chlorine reacts with water in different ways depending on the conditions. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). The equation for this reaction is: Chlorine is only slightly soluble in water, with its maximum solubility. Reaction of chlorine with water. The following equation presents the hydrolysis reaction (usepa. Chlorine With Water Equation.
From www.doubtnut.com
Propene on reaction with chlorine water gives Chlorine With Water Equation Usually, reactions of chlorine with water are for disinfection purposes. Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −. Hclo (aq) → h+ (aq) +. Reaction of chlorine with water. The reaction of chlorine in water is a disproportionation reaction in which the chlorine gets both oxidised and reduced. When chlorine gas is dissolved. Chlorine With Water Equation.
From www.numerade.com
SOLVEDWrite the balanced chemical equation for the reaction of chlorine with water in (a) a Chlorine With Water Equation Chlorine is available in three forms: Chlorine is only slightly soluble in water, with its maximum solubility. Hclo (aq) → h+ (aq) +. The different products have some practical uses. Chlorine reacts with water in different ways depending on the conditions. When chlorine reacts with water, it forms a mixture of hydrochloric acid, hcl, and chloric acid, hclo. The following. Chlorine With Water Equation.
From www.youtube.com
Chlorine Water + Sodium Chloride YouTube Chlorine With Water Equation The chlorine mixed with water will produce hypochlorous acid (hocl). Chlorine is only slightly soluble in water, with its maximum solubility. Usually, reactions of chlorine with water are for disinfection purposes. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). Define disproportionation & describe the role of chlorine in water purification. Use our revision notes to revise the. Chlorine With Water Equation.
From www.chegg.com
Solved 1. Chlorine reacts with water to form an equilibrium Chlorine With Water Equation Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). Define disproportionation & describe the role of chlorine in water purification. Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −. Chlorine reacts with water in different ways depending on the conditions. Usually, reactions of chlorine with water are for disinfection purposes. Chlorine is. Chlorine With Water Equation.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Chlorine With Water Equation The chlorine mixed with water will produce hypochlorous acid (hocl). Chlorine is available in three forms: Cl 2 (g) + h 2 o (l). When chlorine gas is dissolved in water, the reaction forms. Hclo (aq) → h+ (aq) +. Chlorine reacts with water in different ways depending on the conditions. Use our revision notes to revise the reaction of. Chlorine With Water Equation.
From slidetodoc.com
REACTION OF CHLORINE WITH WATER Chlorine dissolves in Chlorine With Water Equation The equation for this reaction is: Chlorine is available in three forms: Chloric (i) acid (hclo) sterilises water by killing bacteria. The different products have some practical uses. Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −. Use our revision notes to revise the reaction of chlorine with naoh. When chlorine gas is dissolved. Chlorine With Water Equation.
From www.chemistryviews.org
The Chemistry of Pools ChemistryViews Chlorine With Water Equation The following equation presents the hydrolysis reaction (usepa 1999; Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −. The different products have some practical uses. Chlorine is only slightly soluble in water, with its maximum solubility. Chlorine is available in three forms: Cl 2 (g) + h 2 o (l). Hclo (aq) → h+. Chlorine With Water Equation.
From www.dreamstime.com
Chlorine Dioxide ClO2 Molecule. Used in Pulp Bleaching and for Disinfection of Drinking Water Chlorine With Water Equation Cl 2 (g) + h 2 o (l). Chloric (i) acid (hclo) sterilises water by killing bacteria. The equation for this reaction is: Define disproportionation & describe the role of chlorine in water purification. The different products have some practical uses. Chlorine reacts with water in different ways depending on the conditions. Chlorine is available in three forms: The following. Chlorine With Water Equation.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Chlorine With Water Equation Cl 2 (g) + h 2 o (l). When chlorine reacts with water, it forms a mixture of hydrochloric acid, hcl, and chloric acid, hclo. Reaction of chlorine with water. When chlorine gas is dissolved in water, the reaction forms. Chlorine is available in three forms: The following equation presents the hydrolysis reaction (usepa 1999; Use our revision notes to. Chlorine With Water Equation.
From www.techquintal.com
10 Advantages and Disadvantages of Chlorination of Water to Know Tech Quintal Chlorine With Water Equation Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −. The different products have some practical uses. Use our revision notes to revise the reaction of chlorine with naoh. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). Chloric (i) acid (hclo) sterilises water by killing bacteria. Chlorine is only slightly soluble in. Chlorine With Water Equation.
From www.youtube.com
How to Balance Cl2 + H2O = HCl + O2 (Chlorine gas + Water) YouTube Chlorine With Water Equation The following equation presents the hydrolysis reaction (usepa 1999; The chlorine mixed with water will produce hypochlorous acid (hocl). Chlorine reacts with water in different ways depending on the conditions. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −. Use our revision notes. Chlorine With Water Equation.
From www.shutterstock.com
Formula And Structure Of Chlorine Dioxide Molecule (Clo2)Main Component Of Mms (Miracle Mineral Chlorine With Water Equation Reaction of chlorine with water. Chloric (i) acid (hclo) sterilises water by killing bacteria. When chlorine gas is dissolved in water, the reaction forms. The following equation presents the hydrolysis reaction (usepa 1999; Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). Cl 2 (g) + h 2 o (l). The equation for this reaction is: Chlorine is. Chlorine With Water Equation.
From www.slideserve.com
PPT Residual Chlorine & Chlorine Demand PowerPoint Presentation ID6595840 Chlorine With Water Equation The different products have some practical uses. The chlorine mixed with water will produce hypochlorous acid (hocl). Usually, reactions of chlorine with water are for disinfection purposes. Chlorine is only slightly soluble in water, with its maximum solubility. Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −. Define disproportionation & describe the role of. Chlorine With Water Equation.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Hydrogen Chloride In Water Equation Chlorine With Water Equation The chlorine mixed with water will produce hypochlorous acid (hocl). Usually, reactions of chlorine with water are for disinfection purposes. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). Reaction of chlorine with water. Chlorine is available in three forms: Chlorine reacts with water in different ways depending on the conditions. The reaction of chlorine in water is. Chlorine With Water Equation.
From elchoroukhost.net
Chemical Equation For Table Salt And Water Elcho Table Chlorine With Water Equation Chlorine reacts with water in different ways depending on the conditions. The equation for this reaction is: Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −. The reaction of chlorine in water is a disproportionation reaction in which the chlorine gets both oxidised and reduced. The different products have some practical uses. Chloric (i). Chlorine With Water Equation.
From stock.adobe.com
Lewis structural formula of chlorine, molecular formula Stock Vector Adobe Stock Chlorine With Water Equation Define disproportionation & describe the role of chlorine in water purification. The following equation presents the hydrolysis reaction (usepa 1999; When chlorine gas is dissolved in water, the reaction forms. Cl 2 (g) + h 2 o (l). The reaction of chlorine in water is a disproportionation reaction in which the chlorine gets both oxidised and reduced. When chlorine reacts. Chlorine With Water Equation.
From www.youtube.com
AQA Further Reactions of Chlorine YouTube Chlorine With Water Equation Usually, reactions of chlorine with water are for disinfection purposes. The reaction of chlorine in water is a disproportionation reaction in which the chlorine gets both oxidised and reduced. The following equation presents the hydrolysis reaction (usepa 1999; Chlorine is only slightly soluble in water, with its maximum solubility. Reaction of chlorine with water. When chlorine gas is dissolved in. Chlorine With Water Equation.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Hydrogen Chloride In Water Equation Chlorine With Water Equation The following equation presents the hydrolysis reaction (usepa 1999; When chlorine reacts with water, it forms a mixture of hydrochloric acid, hcl, and chloric acid, hclo. Define disproportionation & describe the role of chlorine in water purification. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). Usually, reactions of chlorine with water are for disinfection purposes. Hclo (aq). Chlorine With Water Equation.
From www.numerade.com
SOLVEDWrite balanced equations for the reaction of chlorine with the following substances. If Chlorine With Water Equation Hclo (aq) → h+ (aq) +. When chlorine reacts with water, it forms a mixture of hydrochloric acid, hcl, and chloric acid, hclo. Define disproportionation & describe the role of chlorine in water purification. The following equation presents the hydrolysis reaction (usepa 1999; The equation for this reaction is: Chlorine is only slightly soluble in water, with its maximum solubility.. Chlorine With Water Equation.
From www.alamy.com
Chlorine dioxide (ClO2) molecule. Used in pulp bleaching and for disinfection of drinking water Chlorine With Water Equation Hclo (aq) → h+ (aq) +. Define disproportionation & describe the role of chlorine in water purification. Chlorine is only slightly soluble in water, with its maximum solubility. The following equation presents the hydrolysis reaction (usepa 1999; Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −. Chloric (i) acid (hclo) sterilises water by killing. Chlorine With Water Equation.
From www.slideserve.com
PPT Reactions of chlorine with water and sodium hydroxide. PowerPoint Presentation ID3536509 Chlorine With Water Equation When chlorine reacts with water, it forms a mixture of hydrochloric acid, hcl, and chloric acid, hclo. The chlorine mixed with water will produce hypochlorous acid (hocl). Define disproportionation & describe the role of chlorine in water purification. Chlorine is available in three forms: Chlorine reacts with water in different ways depending on the conditions. Chlorine is only slightly soluble. Chlorine With Water Equation.
From www.tessshebaylo.com
Equation For Dissolving Ammonium Chloride In Water Tessshebaylo Chlorine With Water Equation Use our revision notes to revise the reaction of chlorine with naoh. Hclo (aq) → h+ (aq) +. Define disproportionation & describe the role of chlorine in water purification. Reaction of chlorine with water. When chlorine reacts with water, it forms a mixture of hydrochloric acid, hcl, and chloric acid, hclo. The different products have some practical uses. Chlorine is. Chlorine With Water Equation.
From signalticket9.pythonanywhere.com
Out Of This World Iron And Chlorine Reaction Equation University Physics Sheet Chlorine With Water Equation Chlorine is only slightly soluble in water, with its maximum solubility. The reaction of chlorine in water is a disproportionation reaction in which the chlorine gets both oxidised and reduced. Cl 2 (g) + h 2 o (l). Chlorine is available in three forms: Define disproportionation & describe the role of chlorine in water purification. Hclo (aq) → h+ (aq). Chlorine With Water Equation.
From slidetodoc.com
REACTION OF CHLORINE WITH WATER Chlorine dissolves in Chlorine With Water Equation The reaction of chlorine in water is a disproportionation reaction in which the chlorine gets both oxidised and reduced. Use our revision notes to revise the reaction of chlorine with naoh. Cl 2 (g) + h 2 o ⇒ ho cl + h + cl −. Define disproportionation & describe the role of chlorine in water purification. Hclo (aq) →. Chlorine With Water Equation.
From www.youtube.com
Equation for Potassium Chloride Dissolving in Water ( KCl + H2O) YouTube Chlorine With Water Equation Use our revision notes to revise the reaction of chlorine with naoh. Reaction of chlorine with water. Hclo (aq) → h+ (aq) +. The reaction of chlorine in water is a disproportionation reaction in which the chlorine gets both oxidised and reduced. Chlorine reacts with water in different ways depending on the conditions. The following equation presents the hydrolysis reaction. Chlorine With Water Equation.
From socratic.org
What is the chemical equation for HCl dissolving into water and ionizing? Socratic Chlorine With Water Equation The reaction of chlorine in water is a disproportionation reaction in which the chlorine gets both oxidised and reduced. Chlorine is available in three forms: When chlorine gas is dissolved in water, the reaction forms. Chlorine reacts with water in different ways depending on the conditions. Define disproportionation & describe the role of chlorine in water purification. Usually, reactions of. Chlorine With Water Equation.
From www.essentialchemicalindustry.org
Chlorine Chlorine With Water Equation The reaction of chlorine in water is a disproportionation reaction in which the chlorine gets both oxidised and reduced. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). Usually, reactions of chlorine with water are for disinfection purposes. The following equation presents the hydrolysis reaction (usepa 1999; Chlorine is available in three forms: Chlorine reacts with water in. Chlorine With Water Equation.
From ethanatmathis.blogspot.com
Calcium Chloride and Water Equation EthanatMathis Chlorine With Water Equation Reaction of chlorine with water. Chlorine is only slightly soluble in water, with its maximum solubility. Chlorine is available in three forms: When chlorine reacts with water, it forms a mixture of hydrochloric acid, hcl, and chloric acid, hclo. Cl 2 (g) + h 2 o (l). Usually, reactions of chlorine with water are for disinfection purposes. Cl 2 (g). Chlorine With Water Equation.
From brainly.in
hydrogen reacts with chlorine to form hydrogen chloride Brainly.in Chlorine With Water Equation Chlorine reacts with water in different ways depending on the conditions. Define disproportionation & describe the role of chlorine in water purification. The equation for this reaction is: When chlorine gas is dissolved in water, the reaction forms. The reaction of chlorine in water is a disproportionation reaction in which the chlorine gets both oxidised and reduced. Gaseous chlorine (100%. Chlorine With Water Equation.
From www.numerade.com
Chlorine forms from the reaction of hydrochloric acid with manganese(IV) oxide. The balanced Chlorine With Water Equation The following equation presents the hydrolysis reaction (usepa 1999; Reaction of chlorine with water. The equation for this reaction is: Usually, reactions of chlorine with water are for disinfection purposes. Chlorine is only slightly soluble in water, with its maximum solubility. Chlorine reacts with water in different ways depending on the conditions. Hclo (aq) → h+ (aq) +. Cl 2. Chlorine With Water Equation.