How Can Substances Be Classified As Ionic Or Molecular . Predict the type of compound formed from elements based on their location within the. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Define ionic and molecular (covalent) compounds. The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Ionic compounds generally form from metals and nonmetals. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are.
from studylib.net
Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Predict the type of compound formed from elements based on their location within the. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Define ionic and molecular (covalent) compounds. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Ionic compounds generally form from metals and nonmetals. The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are.
Ionic & Covalent Nomenclature PowerPoint
How Can Substances Be Classified As Ionic Or Molecular Compounds that do not contain ions, but instead consist of atoms bonded tightly. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Define ionic and molecular (covalent) compounds. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Predict the type of compound formed from elements based on their location within the. The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Ionic compounds generally form from metals and nonmetals. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms.
From saylordotorg.github.io
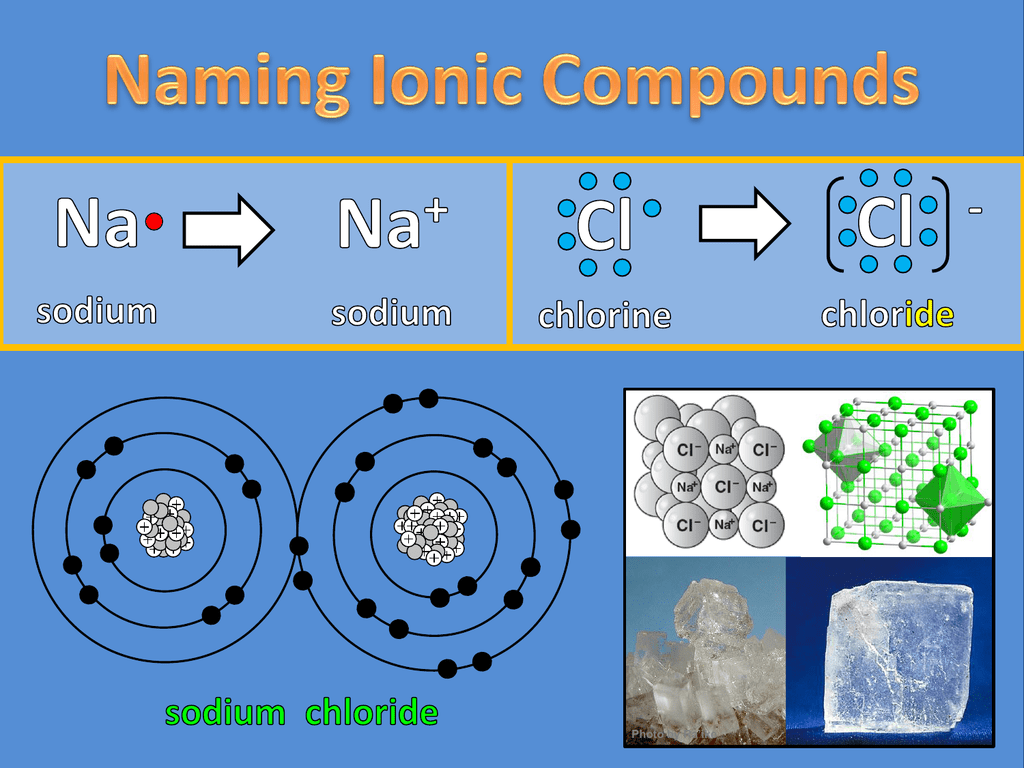
Naming Ionic Compounds How Can Substances Be Classified As Ionic Or Molecular Ionic compounds generally form from metals and nonmetals. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Predict the type of compound formed. How Can Substances Be Classified As Ionic Or Molecular.
From www.slideserve.com
PPT Physical and Chemical Changes Pure Substances Mixtures States of Matter PowerPoint How Can Substances Be Classified As Ionic Or Molecular Predict the type of compound formed from elements based on their location within the. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Compounds that do not contain ions, but instead consist of atoms bonded tightly. The vast majority of known substances can be classified. How Can Substances Be Classified As Ionic Or Molecular.
From wps.prenhall.com
Media Portfolio How Can Substances Be Classified As Ionic Or Molecular Predict the type of compound formed from elements based on their location within the. The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Ionic compounds generally form from metals and nonmetals. Define ionic and molecular (covalent) compounds. Covalent bonds are the attractive forces between the positively charged. How Can Substances Be Classified As Ionic Or Molecular.
From www.youtube.com
Metallic, Ionic, and Molecular Solids Explained with Examples! YouTube How Can Substances Be Classified As Ionic Or Molecular Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Predict the type of compound formed from elements based on their location within the. The vast majority of known substances can be classified by their type of bonding and hence their structures. How Can Substances Be Classified As Ionic Or Molecular.
From www.slideserve.com
PPT Ionic vs. Molecular Compounds PowerPoint Presentation, free download ID3074480 How Can Substances Be Classified As Ionic Or Molecular Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Covalent bonds are the attractive forces between the positively charged. How Can Substances Be Classified As Ionic Or Molecular.
From studylib.net
Ionic Compounds Naming How Can Substances Be Classified As Ionic Or Molecular Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Covalent bonds are the attractive forces between the. How Can Substances Be Classified As Ionic Or Molecular.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination How Can Substances Be Classified As Ionic Or Molecular The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Predict the type of compound formed from elements based on their location within the. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are.. How Can Substances Be Classified As Ionic Or Molecular.
From nandananitisara.blogspot.com
Ionic Vs Molecular Compound Nandana Nitisara How Can Substances Be Classified As Ionic Or Molecular Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Predict the type of compound formed from elements based on their location. How Can Substances Be Classified As Ionic Or Molecular.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? How Can Substances Be Classified As Ionic Or Molecular The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Compounds that do not contain ions, but instead consist of atoms bonded tightly. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Predict. How Can Substances Be Classified As Ionic Or Molecular.
From www.slideserve.com
PPT Chemical Bonds (Ionic and Covalent Bonds) Part 1 PowerPoint Presentation ID3128435 How Can Substances Be Classified As Ionic Or Molecular Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Covalent bonds are the attractive forces between the. How Can Substances Be Classified As Ionic Or Molecular.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There How Can Substances Be Classified As Ionic Or Molecular Ionic compounds generally form from metals and nonmetals. The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Define ionic and molecular (covalent) compounds.. How Can Substances Be Classified As Ionic Or Molecular.
From spmchemistry.blog.onlinetuition.com.my
Physical Properties Ionic Compounds SPM Chemistry How Can Substances Be Classified As Ionic Or Molecular The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Compounds that do not contain ions, but instead consist of atoms bonded tightly. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Predict. How Can Substances Be Classified As Ionic Or Molecular.
From slideplayer.com
Classifying Compounds. ppt download How Can Substances Be Classified As Ionic Or Molecular Predict the type of compound formed from elements based on their location within the. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. The vast majority of known substances can be classified by their type of bonding and hence their structures. How Can Substances Be Classified As Ionic Or Molecular.
From slideplayer.com
Classification of Matter Chapter 3 ppt download How Can Substances Be Classified As Ionic Or Molecular Predict the type of compound formed from elements based on their location within the. Define ionic and molecular (covalent) compounds. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead. How Can Substances Be Classified As Ionic Or Molecular.
From www.dreamstime.com
Ionic and Molecular Compounds Stock Vector Illustration of chemical, electron 210021312 How Can Substances Be Classified As Ionic Or Molecular Compounds that do not contain ions, but instead consist of atoms bonded tightly. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Define. How Can Substances Be Classified As Ionic Or Molecular.
From pediaa.com
Difference Between Ionic and Molecular Compounds How Can Substances Be Classified As Ionic Or Molecular Compounds that do not contain ions, but instead consist of atoms bonded tightly. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more. How Can Substances Be Classified As Ionic Or Molecular.
From www.vectorstock.com
Molecular structures common chemical substances Vector Image How Can Substances Be Classified As Ionic Or Molecular Predict the type of compound formed from elements based on their location within the. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons. How Can Substances Be Classified As Ionic Or Molecular.
From www.slideserve.com
PPT Naming Ionic Compounds PowerPoint Presentation, free download ID2245444 How Can Substances Be Classified As Ionic Or Molecular Compounds that do not contain ions, but instead consist of atoms bonded tightly. Define ionic and molecular (covalent) compounds. The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more. How Can Substances Be Classified As Ionic Or Molecular.
From www.youtube.com
⚗️ Classifying Substances as Atomic, Molecular Elements, Molecular, Ionic Compounds YouTube How Can Substances Be Classified As Ionic Or Molecular Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Covalent bonds are the attractive forces between the positively charged nuclei. How Can Substances Be Classified As Ionic Or Molecular.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry How Can Substances Be Classified As Ionic Or Molecular Compounds that do not contain ions, but instead consist of atoms bonded tightly. Predict the type of compound formed from elements based on their location within the. The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Define ionic and molecular (covalent) compounds. Covalent bonds are the attractive. How Can Substances Be Classified As Ionic Or Molecular.
From www.slideserve.com
PPT Elements and Compounds PowerPoint Presentation, free download ID196981 How Can Substances Be Classified As Ionic Or Molecular Define ionic and molecular (covalent) compounds. Predict the type of compound formed from elements based on their location within the. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. The vast majority of known substances can be classified by their type. How Can Substances Be Classified As Ionic Or Molecular.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica How Can Substances Be Classified As Ionic Or Molecular The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded. How Can Substances Be Classified As Ionic Or Molecular.
From studylib.net
Ionic Compounds How Can Substances Be Classified As Ionic Or Molecular Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Covalent bonds are the attractive forces between the positively charged nuclei of the bonded. How Can Substances Be Classified As Ionic Or Molecular.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica How Can Substances Be Classified As Ionic Or Molecular Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Define ionic and molecular (covalent) compounds. Predict the. How Can Substances Be Classified As Ionic Or Molecular.
From quizizz.com
Properties of ionic compounds Chemistry Quizizz How Can Substances Be Classified As Ionic Or Molecular Compounds that do not contain ions, but instead consist of atoms bonded tightly. Ionic compounds generally form from metals and nonmetals. Define ionic and molecular (covalent) compounds. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Covalent bonds are the attractive forces between the positively. How Can Substances Be Classified As Ionic Or Molecular.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii How Can Substances Be Classified As Ionic Or Molecular The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded. How Can Substances Be Classified As Ionic Or Molecular.
From www.youtube.com
Naming Ionic Compounds, a tutorial Crash Chemistry Academy YouTube How Can Substances Be Classified As Ionic Or Molecular Define ionic and molecular (covalent) compounds. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Predict the. How Can Substances Be Classified As Ionic Or Molecular.
From www.youtube.com
Examples of Ionic Compoiunds YouTube How Can Substances Be Classified As Ionic Or Molecular Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Ionic compounds generally form from metals and nonmetals.. How Can Substances Be Classified As Ionic Or Molecular.
From sciencenotes.org
Compounds With Both Ionic and Covalent Bonds How Can Substances Be Classified As Ionic Or Molecular Define ionic and molecular (covalent) compounds. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Predict the. How Can Substances Be Classified As Ionic Or Molecular.
From en.wikipedia.org
Ionic bonding Wikipedia How Can Substances Be Classified As Ionic Or Molecular The vast majority of known substances can be classified by their type of bonding and hence their structures into four main categories; Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Ionic compounds generally form from metals and nonmetals. Covalent bonds are the attractive forces. How Can Substances Be Classified As Ionic Or Molecular.
From sciencenotes.org
Ionic Compound Properties How Can Substances Be Classified As Ionic Or Molecular Ionic compounds generally form from metals and nonmetals. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Covalent bonds are the. How Can Substances Be Classified As Ionic Or Molecular.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts How Can Substances Be Classified As Ionic Or Molecular Predict the type of compound formed from elements based on their location within the. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or. How Can Substances Be Classified As Ionic Or Molecular.
From studylib.net
Ionic & Covalent Nomenclature PowerPoint How Can Substances Be Classified As Ionic Or Molecular Define ionic and molecular (covalent) compounds. Ionic compounds generally form from metals and nonmetals. Predict the type of compound formed from elements based on their location within the. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. The vast majority of known substances can be. How Can Substances Be Classified As Ionic Or Molecular.
From sciencenotes.org
Examples of Ionic Compounds in Everyday Life How Can Substances Be Classified As Ionic Or Molecular Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Predict the type of compound formed from elements based on their location within the. Covalent bonds are the attractive forces between the positively. How Can Substances Be Classified As Ionic Or Molecular.
From chem.libretexts.org
6.2 Comparing Ionic and Molecular Substances Chemistry LibreTexts How Can Substances Be Classified As Ionic Or Molecular Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Ionic compounds generally form from metals and nonmetals.. How Can Substances Be Classified As Ionic Or Molecular.