Beer's Law And Spectrophotometric Analysis Lab Report . The concentration (in ppm) of an unknown sample of iron will. (1) where k 2 is beer’s proportionality constant, a is. Introduction to spectrophotometer and beer’s law procedure: Determine the concentration of copper(ii) ions in solution. This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. You will determine the concentration of an. Beer’s law can be described by the following expression [standard methods, 1992]: Use the beer's law plot to determine the concentration of an unknown analyte. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calibrate a spectrometer and create a linear beer's law plot; Beer’s law fabrizio chigne valerga lab partner: This relationship is called the. The pre lab report on page 22 of my lab notebook shows the general procedure used.
from www.studocu.com
The pre lab report on page 22 of my lab notebook shows the general procedure used. Introduction to spectrophotometer and beer’s law procedure: The concentration (in ppm) of an unknown sample of iron will. You will determine the concentration of an. This relationship is called the. Calibrate a spectrometer and create a linear beer's law plot; (1) where k 2 is beer’s proportionality constant, a is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer’s law fabrizio chigne valerga lab partner: Beer’s law can be described by the following expression [standard methods, 1992]:
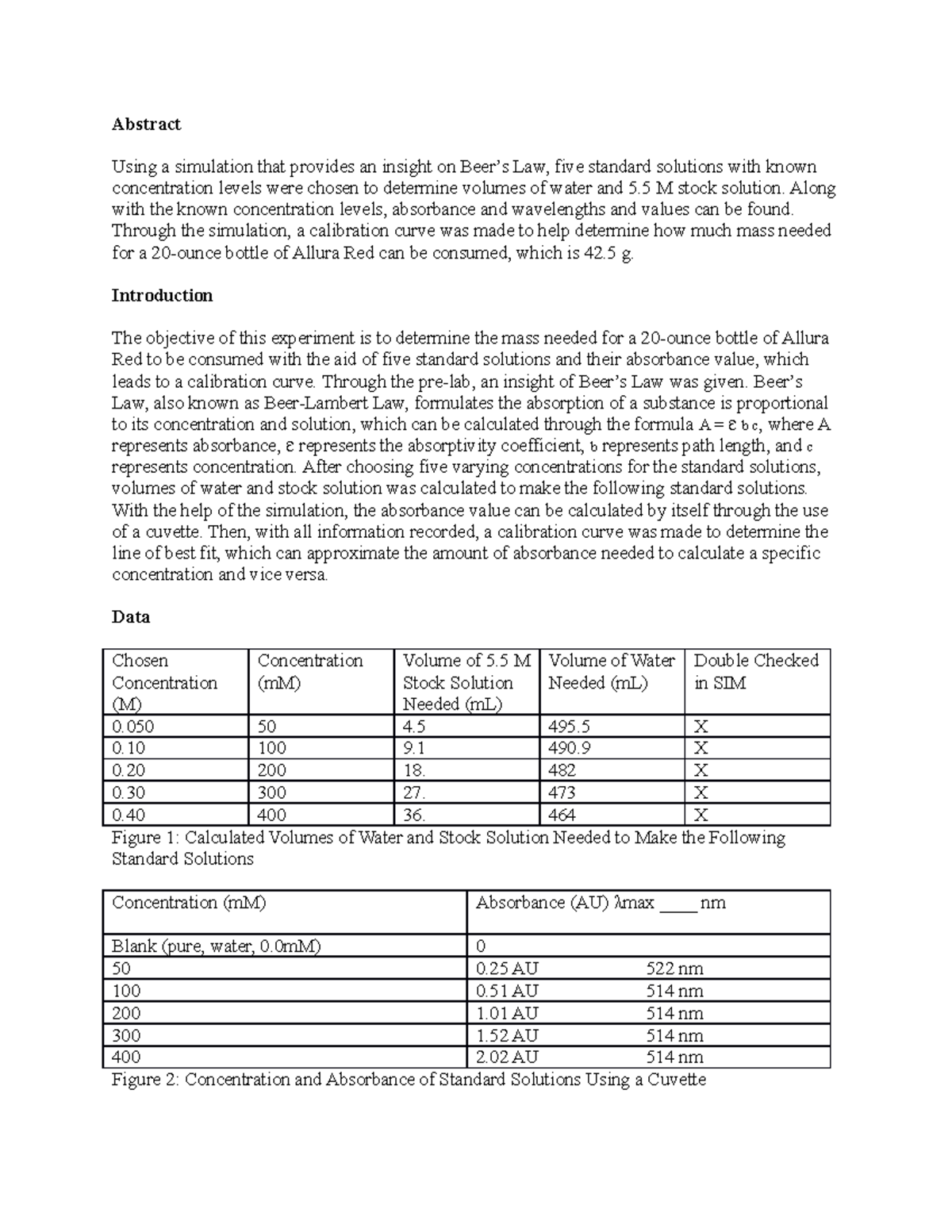
Chem 227 Beer's Law Lap report of beer lab Abstract Using a
Beer's Law And Spectrophotometric Analysis Lab Report You will determine the concentration of an. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The pre lab report on page 22 of my lab notebook shows the general procedure used. You will determine the concentration of an. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Beer’s law can be described by the following expression [standard methods, 1992]: Use the beer's law plot to determine the concentration of an unknown analyte. Determine the concentration of copper(ii) ions in solution. Introduction to spectrophotometer and beer’s law procedure: This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. The concentration (in ppm) of an unknown sample of iron will. This relationship is called the. (1) where k 2 is beer’s proportionality constant, a is. Calibrate a spectrometer and create a linear beer's law plot; Beer’s law fabrizio chigne valerga lab partner:
From www.studocu.com
Chem 227 Beer's Law Lap report of beer lab Abstract Using a Beer's Law And Spectrophotometric Analysis Lab Report Beer’s law fabrizio chigne valerga lab partner: Beer’s law can be described by the following expression [standard methods, 1992]: Introduction to spectrophotometer and beer’s law procedure: The concentration (in ppm) of an unknown sample of iron will. Calibrate a spectrometer and create a linear beer's law plot; This relationship is called the. This lab will teach one how to properly. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.thinkswap.com
Lab Report Beer's law and standard curve for coloured compounds BMC Beer's Law And Spectrophotometric Analysis Lab Report Use the beer's law plot to determine the concentration of an unknown analyte. The concentration (in ppm) of an unknown sample of iron will. Beer’s law can be described by the following expression [standard methods, 1992]: The pre lab report on page 22 of my lab notebook shows the general procedure used. The amount of light that a species absorbs. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.studocu.com
Experiment 1 Spectrophotometry and the BeerLambert Law Experiment 1 Beer's Law And Spectrophotometric Analysis Lab Report Determine the concentration of copper(ii) ions in solution. This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Use the beer's law plot to determine. Beer's Law And Spectrophotometric Analysis Lab Report.
From studylib.net
Beer's_Law_Lab_with_Analysis Beer's Law And Spectrophotometric Analysis Lab Report This relationship is called the. Determine the concentration of copper(ii) ions in solution. Beer’s law can be described by the following expression [standard methods, 1992]: The concentration (in ppm) of an unknown sample of iron will. Use the beer's law plot to determine the concentration of an unknown analyte. You will determine the concentration of an. Introduction to spectrophotometer and. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.studocu.com
Beer’s Law and Spectrophotometry Lab Experiment Title Beer’s Law and Beer's Law And Spectrophotometric Analysis Lab Report Beer’s law can be described by the following expression [standard methods, 1992]: Calibrate a spectrometer and create a linear beer's law plot; Use the beer's law plot to determine the concentration of an unknown analyte. Determine the concentration of copper(ii) ions in solution. (1) where k 2 is beer’s proportionality constant, a is. The direct relationship between absorbance and concentration. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.chegg.com
Solved Experiment 12 Beer's Law PreLab Report Sheet Name Beer's Law And Spectrophotometric Analysis Lab Report Determine the concentration of copper(ii) ions in solution. This relationship is called the. You will determine the concentration of an. The concentration (in ppm) of an unknown sample of iron will. The pre lab report on page 22 of my lab notebook shows the general procedure used. The direct relationship between absorbance and concentration for a solution is known as. Beer's Law And Spectrophotometric Analysis Lab Report.
From studylib.net
Lab 4 Determination of Solution Concentration Using Beer's Law Beer's Law And Spectrophotometric Analysis Lab Report The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. (1) where k 2 is beer’s proportionality constant, a is. The pre lab report on page 22 of my lab notebook shows the general procedure used. This relationship is called the. The direct relationship between absorbance and concentration. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.physicsforums.com
Spectrophotometric Determination of Fe2+ (Beer's Law) Beer's Law And Spectrophotometric Analysis Lab Report This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. Use the beer's law plot to determine the concentration of an unknown analyte. Calibrate a spectrometer and create a linear beer's law plot; Beer’s law fabrizio chigne valerga lab partner: Determine the concentration of copper(ii) ions in. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.chegg.com
Solved Beer's Law lab Answer & Show work please Part A 1) Beer's Law And Spectrophotometric Analysis Lab Report You will determine the concentration of an. Use the beer's law plot to determine the concentration of an unknown analyte. The pre lab report on page 22 of my lab notebook shows the general procedure used. This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. The. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.chegg.com
Solved Spectrophotometry and Beer's Law spremenities Lab Beer's Law And Spectrophotometric Analysis Lab Report (1) where k 2 is beer’s proportionality constant, a is. Determine the concentration of copper(ii) ions in solution. The concentration (in ppm) of an unknown sample of iron will. This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. Beer’s law fabrizio chigne valerga lab partner: Introduction. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Beer's Law And Spectrophotometric Analysis Lab Report This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The pre lab report on page 22 of my lab notebook shows the general procedure used. This relationship is called the. Introduction. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.studocu.com
CHE144 Lab Manual Experiments 1 EXPERIMENT ONE Spectrophotometry and Beer's Law And Spectrophotometric Analysis Lab Report The concentration (in ppm) of an unknown sample of iron will. This relationship is called the. You will determine the concentration of an. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Introduction to spectrophotometer and beer’s law procedure: (1) where k 2 is beer’s proportionality constant,. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.studypool.com
SOLUTION Spectrophotometry , spectrum, absorption Beer's Law And Spectrophotometric Analysis Lab Report The pre lab report on page 22 of my lab notebook shows the general procedure used. This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. You will determine the concentration of. Beer's Law And Spectrophotometric Analysis Lab Report.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law And Spectrophotometric Analysis Lab Report The direct relationship between absorbance and concentration for a solution is known as beer’s law. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer’s law fabrizio chigne valerga lab partner: This lab will teach one how to properly set up and operate a spectrophotometer, as well. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.scribd.com
Determination of the Concentration of an Unknown Solution Through Beer's Law And Spectrophotometric Analysis Lab Report This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Calibrate a spectrometer and create a linear beer's law plot; The amount of light that a species absorbs in a spectroscopic transition. Beer's Law And Spectrophotometric Analysis Lab Report.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Beer's Law And Spectrophotometric Analysis Lab Report The direct relationship between absorbance and concentration for a solution is known as beer’s law. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The pre lab report on page 22 of my lab notebook shows the general procedure used. Use the beer's law plot to determine. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.youtube.com
Exp. 18 Spectrophotometric Analysis Concentration of a Solution Beer's Law And Spectrophotometric Analysis Lab Report (1) where k 2 is beer’s proportionality constant, a is. Beer’s law fabrizio chigne valerga lab partner: The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The concentration (in ppm) of an unknown sample of iron will. You will determine the concentration of an. The pre lab. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.scribd.com
beers law lab report example Molar Concentration Concentration Beer's Law And Spectrophotometric Analysis Lab Report The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The concentration (in ppm) of an unknown sample of iron will. This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. The direct relationship between. Beer's Law And Spectrophotometric Analysis Lab Report.
From studylib.net
Beer's Law Lab Beer's Law And Spectrophotometric Analysis Lab Report Determine the concentration of copper(ii) ions in solution. Beer’s law fabrizio chigne valerga lab partner: This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. You will determine the concentration of an. Introduction to spectrophotometer and beer’s law procedure: The concentration (in ppm) of an unknown sample. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.scribd.com
Spectrophotometric Methods Beer's Law Analysis PDF Beer's Law And Spectrophotometric Analysis Lab Report This relationship is called the. (1) where k 2 is beer’s proportionality constant, a is. Beer’s law can be described by the following expression [standard methods, 1992]: This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. Determine the concentration of copper(ii) ions in solution. You will. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.transtutors.com
(Solved) Spectrophotometry And Beer's Law Concentration Of Cr' In Beer's Law And Spectrophotometric Analysis Lab Report The concentration (in ppm) of an unknown sample of iron will. Introduction to spectrophotometer and beer’s law procedure: The direct relationship between absorbance and concentration for a solution is known as beer’s law. Use the beer's law plot to determine the concentration of an unknown analyte. Beer’s law fabrizio chigne valerga lab partner: This lab will teach one how to. Beer's Law And Spectrophotometric Analysis Lab Report.
From studylib.net
Beer's Law Lab Beer's Law And Spectrophotometric Analysis Lab Report This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. You will determine the concentration of an. Introduction to spectrophotometer and beer’s law procedure: Calibrate a spectrometer and create a linear beer's law plot; Determine the concentration of copper(ii) ions in solution. Beer’s law fabrizio chigne valerga. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.studypool.com
SOLUTION Spectrophotometry , spectrum, absorption Beer's Law And Spectrophotometric Analysis Lab Report The direct relationship between absorbance and concentration for a solution is known as beer’s law. The pre lab report on page 22 of my lab notebook shows the general procedure used. Calibrate a spectrometer and create a linear beer's law plot; Beer’s law can be described by the following expression [standard methods, 1992]: Beer’s law fabrizio chigne valerga lab partner:. Beer's Law And Spectrophotometric Analysis Lab Report.
From stemfinity.com
Spectrophotometric Analysis of Copper Beer Law Kemtec Science Beer's Law And Spectrophotometric Analysis Lab Report Beer’s law fabrizio chigne valerga lab partner: Use the beer's law plot to determine the concentration of an unknown analyte. Beer’s law can be described by the following expression [standard methods, 1992]: The direct relationship between absorbance and concentration for a solution is known as beer’s law. You will determine the concentration of an. (1) where k 2 is beer’s. Beer's Law And Spectrophotometric Analysis Lab Report.
From studylib.net
Casestudy The BeerLambert Law and Spectrophotometry Learning objectives Beer's Law And Spectrophotometric Analysis Lab Report Beer’s law can be described by the following expression [standard methods, 1992]: Introduction to spectrophotometer and beer’s law procedure: The direct relationship between absorbance and concentration for a solution is known as beer’s law. This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. The concentration (in. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.youtube.com
Spectrophotometric terms and BeerLambert Law YouTube Beer's Law And Spectrophotometric Analysis Lab Report Introduction to spectrophotometer and beer’s law procedure: The concentration (in ppm) of an unknown sample of iron will. This relationship is called the. (1) where k 2 is beer’s proportionality constant, a is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer’s law can be described. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.youtube.com
Spectrophotometry BeerLambert Law. YouTube Beer's Law And Spectrophotometric Analysis Lab Report Beer’s law can be described by the following expression [standard methods, 1992]: Beer’s law fabrizio chigne valerga lab partner: This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. The concentration (in ppm) of an unknown sample of iron will. (1) where k 2 is beer’s proportionality. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.studocu.com
Beer's Law Lab Beer's Law, 20192020 academic year Experiment 4 Beer's Law And Spectrophotometric Analysis Lab Report The pre lab report on page 22 of my lab notebook shows the general procedure used. Use the beer's law plot to determine the concentration of an unknown analyte. Beer’s law can be described by the following expression [standard methods, 1992]: (1) where k 2 is beer’s proportionality constant, a is. Beer’s law fabrizio chigne valerga lab partner: This relationship. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.studocu.com
Spectrophotometry Lab MT Virtual Lab Report Spectrophotometry Learn Beer's Law And Spectrophotometric Analysis Lab Report Calibrate a spectrometer and create a linear beer's law plot; Beer’s law fabrizio chigne valerga lab partner: The pre lab report on page 22 of my lab notebook shows the general procedure used. Use the beer's law plot to determine the concentration of an unknown analyte. The direct relationship between absorbance and concentration for a solution is known as beer’s. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.chegg.com
EXPERIMENT 5 SPECTROPHOTOMETRY AND BEER'S LAW The Beer's Law And Spectrophotometric Analysis Lab Report Calibrate a spectrometer and create a linear beer's law plot; The direct relationship between absorbance and concentration for a solution is known as beer’s law. Beer’s law fabrizio chigne valerga lab partner: The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This lab will teach one how. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.chegg.com
Solved SPECTROPHOTOMETRY • APPLY BEER'S LAW TO DETERMINE Beer's Law And Spectrophotometric Analysis Lab Report You will determine the concentration of an. The direct relationship between absorbance and concentration for a solution is known as beer’s law. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. (1) where k 2 is beer’s proportionality constant, a is. Use. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.physicsforums.com
Spectrophotometric Determination of Fe2+ (Beer's Law) Beer's Law And Spectrophotometric Analysis Lab Report Determine the concentration of copper(ii) ions in solution. The direct relationship between absorbance and concentration for a solution is known as beer’s law. This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. You will determine the concentration of an. This relationship is called the. The concentration. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law And Spectrophotometric Analysis Lab Report (1) where k 2 is beer’s proportionality constant, a is. Introduction to spectrophotometer and beer’s law procedure: The concentration (in ppm) of an unknown sample of iron will. Beer’s law fabrizio chigne valerga lab partner: You will determine the concentration of an. Beer’s law can be described by the following expression [standard methods, 1992]: This lab will teach one how. Beer's Law And Spectrophotometric Analysis Lab Report.
From www.studocu.com
Lab report Experiment 2. Spectroscopic Analysis using Beer’s Law Beer's Law And Spectrophotometric Analysis Lab Report Use the beer's law plot to determine the concentration of an unknown analyte. Beer’s law fabrizio chigne valerga lab partner: Determine the concentration of copper(ii) ions in solution. You will determine the concentration of an. This lab will teach one how to properly set up and operate a spectrophotometer, as well as apply the principle of beer’s law. The concentration. Beer's Law And Spectrophotometric Analysis Lab Report.
From future4200.com
Getting started in analytical chemistry Spectrophotometry and Analysis Beer's Law And Spectrophotometric Analysis Lab Report The direct relationship between absorbance and concentration for a solution is known as beer’s law. Introduction to spectrophotometer and beer’s law procedure: Beer’s law can be described by the following expression [standard methods, 1992]: The pre lab report on page 22 of my lab notebook shows the general procedure used. The amount of light that a species absorbs in a. Beer's Law And Spectrophotometric Analysis Lab Report.