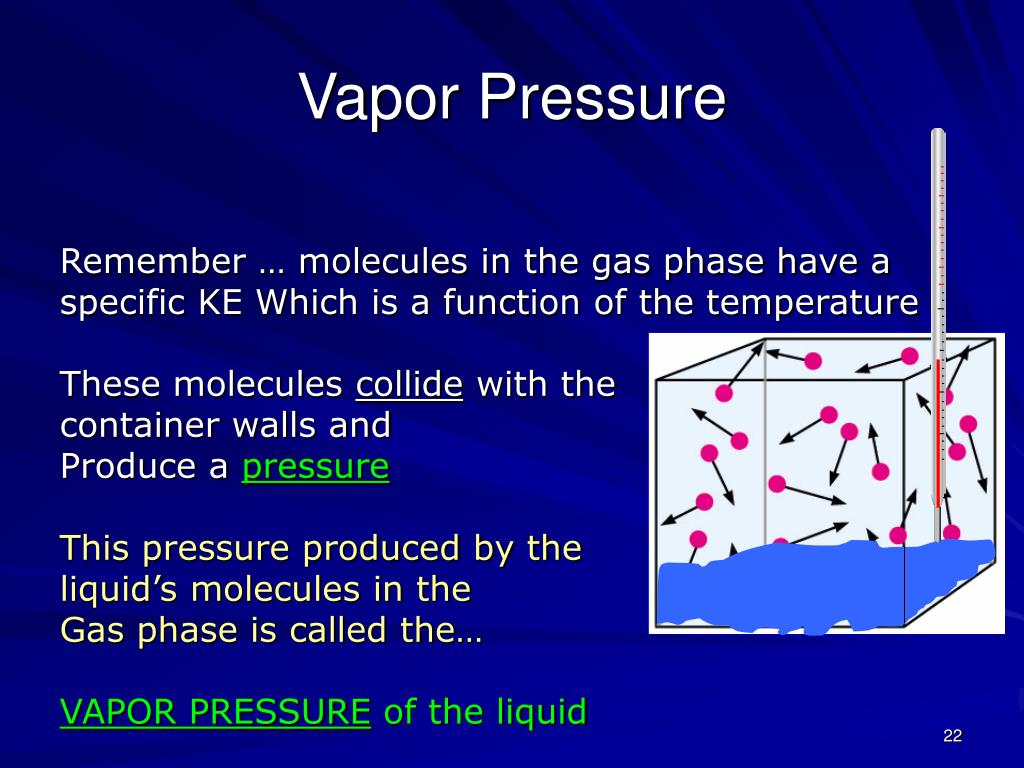
Vapor Point Definition . When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. The temperature at which a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. Boiling is the rapid vaporization of.
from www.slideserve.com
Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Boiling is the rapid vaporization of. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. The temperature at which a.
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574
Vapor Point Definition Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Boiling is the rapid vaporization of. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. The temperature at which a. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download Vapor Point Definition This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. Boiling is the rapid vaporization of. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a. Vapor Point Definition.
From sciencenotes.org
What Is Dew Point? Vapor Point Definition Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The temperature at which a. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. This page looks at how the equilibrium between a liquid (or a solid) and its. Vapor Point Definition.
From mavink.com
Vapor Pressure And Boiling Point Relationship Vapor Point Definition Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The temperature at which a. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. This page looks at how the equilibrium between a liquid (or a solid) and its. Vapor Point Definition.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download Vapor Point Definition Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Boiling is the rapid vaporization of. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given. Vapor Point Definition.
From socratic.org
What is the relation between critical temperature and boiling point or Vapor Point Definition Boiling is the rapid vaporization of. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. The vapor pressure of a. Vapor Point Definition.
From www.vrogue.co
Physical Equilibrium Vapor Pressure And Boiling Point vrogue.co Vapor Point Definition The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Boiling is the. Vapor Point Definition.
From www.chegg.com
Solved Four vapor pressure data pointstwo representing Vapor Point Definition Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. Boiling is the rapid vaporization of. The temperature at which a. Vapour pressure, pressure exerted by a vapour when the vapour is in. Vapor Point Definition.
From vaporpoint.net
A Brief History Vapor Point Leading the Way in Emission Reduction Vapor Point Definition The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The temperature at. Vapor Point Definition.
From courses.lumenlearning.com
Phase Diagrams Chemistry Vapor Point Definition Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Boiling is the rapid vaporization of. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. The temperature at which a.. Vapor Point Definition.
From www.researchgate.net
Pressure retrieved versus actual measurements at water vapor point Vapor Point Definition Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea. Vapor Point Definition.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Vapor Point Definition Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. When the vapor pressure equals the external pressure, bubbles of vapor form within the. Vapor Point Definition.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Vapor Point Definition This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The temperature at which a. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils.. Vapor Point Definition.
From enginedbcentesimal.z21.web.core.windows.net
Phase Diagram 2 Component Vapor Point Definition The temperature at which a. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form,. Vapor Point Definition.
From www.vrogue.co
Physical Equilibrium Vapor Pressure And Boiling Point vrogue.co Vapor Point Definition Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Boiling is the. Vapor Point Definition.
From www.youtube.com
Vapor Pressure Normal Boiling Point & Clausius Clapeyron Equation Vapor Point Definition Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Vapor pressure is. Vapor Point Definition.
From www.slideserve.com
PPT Periodic Table Trends Melting & Boiling Point PowerPoint Vapor Point Definition Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Boiling is the rapid vaporization of. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. When. Vapor Point Definition.
From mungfali.com
Vapor Pressure And Boiling Point Relationship Vapor Point Definition Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. The temperature at which a. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils.. Vapor Point Definition.
From study.com
Vapor Pressure Definition, Formula & Examples Lesson Vapor Point Definition This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. The temperature at which a. Boiling is the rapid vaporization of. Vapour pressure, pressure exerted by a vapour when the. Vapor Point Definition.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Vapor Point Definition Boiling is the rapid vaporization of. This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases. Vapor Point Definition.
From www.youtube.com
Physical Vapour Deposition Detailed Explanation YouTube Vapor Point Definition This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. The temperature at which a. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The vapor pressure of a liquid is the equilibrium. Vapor Point Definition.
From www.youtube.com
Which Compound Has a Higher Vapor Pressure? Intermolecular Force Vapor Point Definition Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. The vapor pressure of a. Vapor Point Definition.
From www.youtube.com
what is vapor pressure and flash point of crude oil? YouTube Vapor Point Definition Boiling is the rapid vaporization of. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The temperature at which a. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. This page looks at how the equilibrium between a liquid. Vapor Point Definition.
From www.scribd.com
Vapor Pressure Boiling Points PDF Vapor Point Definition Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The temperature at which a. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid,. Vapor Point Definition.
From sciencenotes.org
Triple Point Definition Triple Point of Water Vapor Point Definition Boiling is the rapid vaporization of. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. Vapour pressure,. Vapor Point Definition.
From www.youtube.com
Intermolecular Forces Trends Melting & Boiling Point, Viscosity Vapor Point Definition Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Boiling is the rapid vaporization of. The temperature at which a. This page looks at how the equilibrium between a. Vapor Point Definition.
From serc.carleton.edu
Phase Rule Vapor Point Definition The temperature at which a. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both,. Vapor Point Definition.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Vapor Point Definition This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. The temperature. Vapor Point Definition.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Vapor Point Definition Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. Boiling is the rapid vaporization of. Generally a substance's vapor pressure increases as temperature. Vapor Point Definition.
From www.vrogue.co
Physical Equilibrium Vapor Pressure And Boiling Point vrogue.co Vapor Point Definition Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. Boiling is the rapid vaporization of. The temperature at which a.. Vapor Point Definition.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID Vapor Point Definition Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. Boiling is the rapid. Vapor Point Definition.
From chemistnotes.com
Boiling Point Definition, Boiling point as physical properties Vapor Point Definition Boiling is the rapid vaporization of. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This page looks at how the equilibrium between a liquid (or a solid) and its. Vapor Point Definition.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Vapor Point Definition Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Boiling is the rapid vaporization of. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both,. Vapor Point Definition.
From www.youtube.com
Simplest Way To Understand Boiling Point & Vapor Pressure YouTube Vapor Point Definition This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. When the vapor pressure equals the external pressure, bubbles of vapor form within the liquid, and it boils. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. The temperature. Vapor Point Definition.
From ar.inspiredpencil.com
Heat Of Vaporization Equation Vapor Point Definition Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the. Vapor Point Definition.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2237600 Vapor Point Definition This page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Boiling is the rapid vaporization of. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or. Vapor Point Definition.