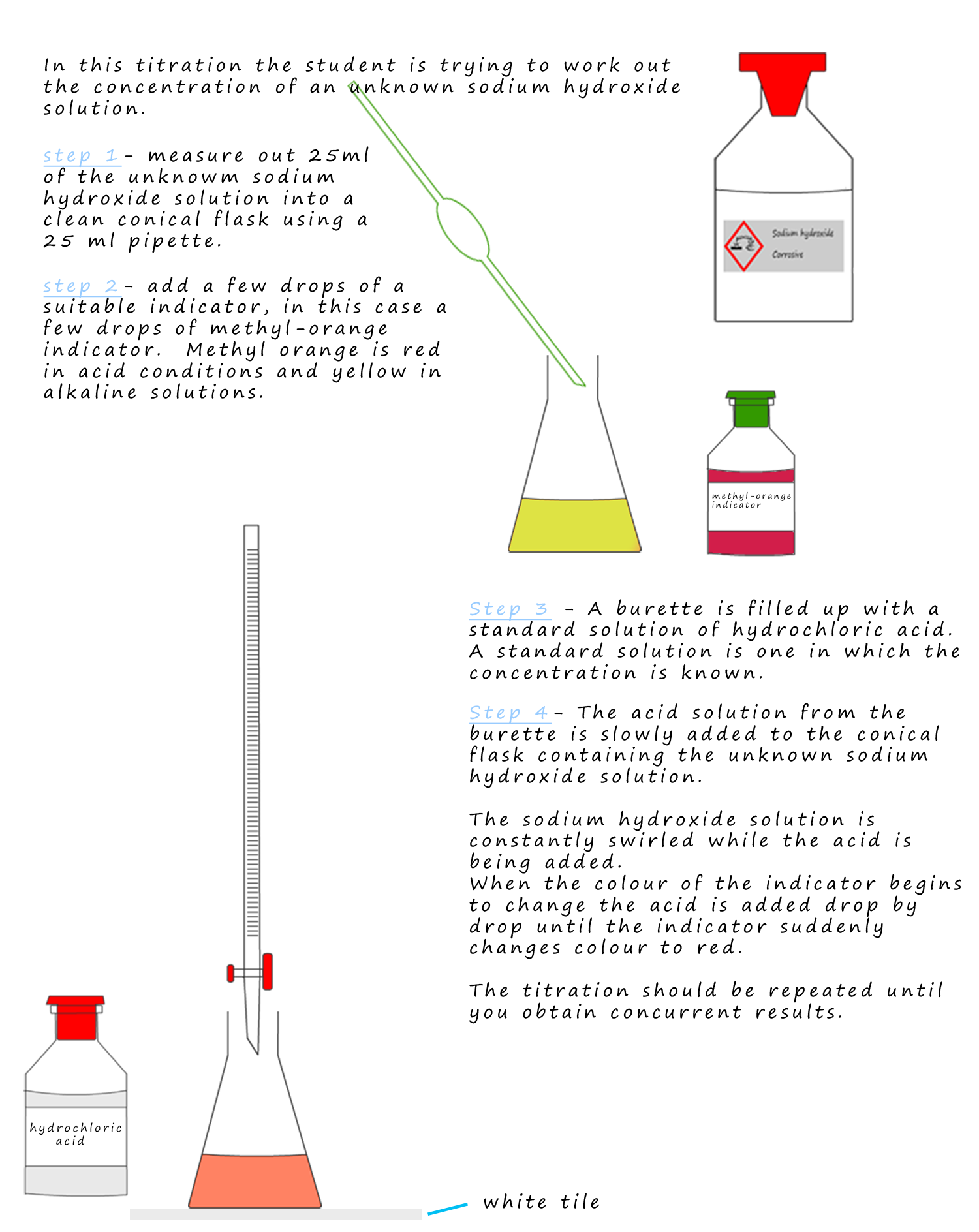
Why Is A Pipette Used To Measure Sodium Hydroxide . Use microscale titration to complete an. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. In equation 1, the acid is hcl (called. Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. In association with nuffield foundation. The pipette allows the same volume of acid to be added. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water.
from www.science-revision.co.uk
Explain why a pipette is used to measure the acid, rather than a measuring cylinder. In association with nuffield foundation. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. Use microscale titration to complete an. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the. The pipette allows the same volume of acid to be added. In equation 1, the acid is hcl (called. Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask.
Titrations
Why Is A Pipette Used To Measure Sodium Hydroxide Explain why a pipette is used to measure the acid, rather than a measuring cylinder. The pipette allows the same volume of acid to be added. Use microscale titration to complete an. Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. In equation 1, the acid is hcl (called. In association with nuffield foundation. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask.
From www.pipettes.com
Types of Pipettes and How to Use Them Why Is A Pipette Used To Measure Sodium Hydroxide The pipette allows the same volume of acid to be added. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask. Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. In this experiment, you will use the acid (potassium hydrogen phthalate. Why Is A Pipette Used To Measure Sodium Hydroxide.
From fyoyxlhue.blob.core.windows.net
Why Use A Pipette In Titration at Grant blog Why Is A Pipette Used To Measure Sodium Hydroxide In association with nuffield foundation. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask. In equation 1, the acid is hcl (called. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. Explain why. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.alamy.com
Titration sodium hydroxide High Resolution Stock Photography and Images Why Is A Pipette Used To Measure Sodium Hydroxide Explain why a pipette is used to measure the acid, rather than a measuring cylinder. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the. Explain why a pipette is used to measure the alkali, rather. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.microlit.us
What is a Micropipette, Types, How to use, Pipetting Techniques Why Is A Pipette Used To Measure Sodium Hydroxide One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. In equation 1, the acid is hcl (called. Use microscale titration to complete an. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. Using a volumetric pipet, measure out 25.00 ml of. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.microlit.us
Uses of Burettes in Pharmaceutical Industry Diagrams & Functions Why Is A Pipette Used To Measure Sodium Hydroxide One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. In association with nuffield foundation. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.alamy.com
Titration sodium hydroxide High Resolution Stock Photography and Images Why Is A Pipette Used To Measure Sodium Hydroxide In equation 1, the acid is hcl (called. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the. The pipette allows the same volume of acid to be added. In association with nuffield foundation. Explain why. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.globalgilson.com
Measuring Pipette (Mohr Type) with Graduations Gilson Co. Why Is A Pipette Used To Measure Sodium Hydroxide In equation 1, the acid is hcl (called. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. Explain why a. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.alamy.com
Rack with test tubes, plastic bottle, pipette vials with chemicals Why Is A Pipette Used To Measure Sodium Hydroxide In equation 1, the acid is hcl (called. Use microscale titration to complete an. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. Using a volumetric pipet, measure out 25.00 ml of. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.tutormyself.com
233 Archives TutorMyself Chemistry Why Is A Pipette Used To Measure Sodium Hydroxide Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask. The pipette allows the same volume of acid to be added. In equation 1, the acid is hcl (called. In association with nuffield foundation. Explain why a pipette is used to measure the acid, rather than a. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.gilson.com
Gilson Article Pipetting Small Volumes 4 Tips for Improving Your Why Is A Pipette Used To Measure Sodium Hydroxide In equation 1, the acid is hcl (called. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.alamy.com
Titration sodium hydroxide High Resolution Stock Photography and Images Why Is A Pipette Used To Measure Sodium Hydroxide The pipette allows the same volume of acid to be added. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. Use microscale titration to complete an. In this experiment, you will use. Why Is A Pipette Used To Measure Sodium Hydroxide.
From microbenotes.com
Pipette Principle, Parts, Types, Procedure, Uses, Examples Why Is A Pipette Used To Measure Sodium Hydroxide Use microscale titration to complete an. The pipette allows the same volume of acid to be added. Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. In association with nuffield foundation. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask.. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.wikihow.com
How to Pipette 14 Steps (with Pictures) wikiHow Why Is A Pipette Used To Measure Sodium Hydroxide Use microscale titration to complete an. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. In association with nuffield foundation. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask. One type of titration uses a neutralization reaction, in which an. Why Is A Pipette Used To Measure Sodium Hydroxide.
From studylib.net
Lab 1 Using the Pipet Why Is A Pipette Used To Measure Sodium Hydroxide The pipette allows the same volume of acid to be added. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. In equation 1, the acid is hcl (called. One type of titration uses a neutralization reaction, in which an. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.vrogue.co
How To Use A Micropipette Pipette Com vrogue.co Why Is A Pipette Used To Measure Sodium Hydroxide Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. Use microscale titration to complete an. In. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.integra-biosciences.com
Everything you need to know about pipettes INTEGRA Why Is A Pipette Used To Measure Sodium Hydroxide Explain why a pipette is used to measure the acid, rather than a measuring cylinder. In association with nuffield foundation. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar. Why Is A Pipette Used To Measure Sodium Hydroxide.
From americanhistory.si.edu
Mohr Measuring Pipette National Museum of American History Why Is A Pipette Used To Measure Sodium Hydroxide Explain why a pipette is used to measure the acid, rather than a measuring cylinder. In equation 1, the acid is hcl (called. Use microscale titration to complete an. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be. Why Is A Pipette Used To Measure Sodium Hydroxide.
From mavink.com
Sodium Hydroxide Ph Chart Why Is A Pipette Used To Measure Sodium Hydroxide The pipette allows the same volume of acid to be added. In association with nuffield foundation. Use microscale titration to complete an. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask. In equation 1, the acid is hcl (called. One type of titration uses a neutralization. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.alamy.com
Alkali dissolving polyester. A pipette is used to put a drop of Why Is A Pipette Used To Measure Sodium Hydroxide The pipette allows the same volume of acid to be added. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. In association with nuffield foundation. Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.labmate-online.com
Sodium Hydroxide Concentration Measure in Less than 10 Seconds Labmate Why Is A Pipette Used To Measure Sodium Hydroxide Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the. One type of. Why Is A Pipette Used To Measure Sodium Hydroxide.
From teachernotes4u.com
Diagram shows the apparatus setup to study the reaction between sodium Why Is A Pipette Used To Measure Sodium Hydroxide Explain why a pipette is used to measure the acid, rather than a measuring cylinder. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the. Use microscale titration to complete an. In association with nuffield foundation.. Why Is A Pipette Used To Measure Sodium Hydroxide.
From mavink.com
Titration Pipette Why Is A Pipette Used To Measure Sodium Hydroxide In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. Explain why a pipette. Why Is A Pipette Used To Measure Sodium Hydroxide.
From stock.adobe.com
Foto de Inscription Sodium hydroxide next to the hand of a laboratory Why Is A Pipette Used To Measure Sodium Hydroxide In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the. Use microscale titration to complete an. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. One type of titration uses. Why Is A Pipette Used To Measure Sodium Hydroxide.
From chemed.chem.purdue.edu
Picture of a Pipet Why Is A Pipette Used To Measure Sodium Hydroxide In association with nuffield foundation. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. In equation 1, the acid is hcl (called. The pipette allows the same volume of acid to be added. Explain why a pipette is used to measure the acid, rather than a measuring. Why Is A Pipette Used To Measure Sodium Hydroxide.
From solutions.pipette.com
Proper Pipetting Resources Pipette Solutions Why Is A Pipette Used To Measure Sodium Hydroxide Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.science-revision.co.uk
Titrations Why Is A Pipette Used To Measure Sodium Hydroxide One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. Use microscale titration to complete an. In association with nuffield foundation. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask. Explain why a pipette. Why Is A Pipette Used To Measure Sodium Hydroxide.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Why Is A Pipette Used To Measure Sodium Hydroxide Explain why a pipette is used to measure the acid, rather than a measuring cylinder. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. The pipette allows the same volume of acid. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.numerade.com
SOLVED Suppose that you used the wrong pipette and dispensed 5.00 mL Why Is A Pipette Used To Measure Sodium Hydroxide In equation 1, the acid is hcl (called. In association with nuffield foundation. Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar. Why Is A Pipette Used To Measure Sodium Hydroxide.
From exogatbyy.blob.core.windows.net
Volumetric Flask Used In Titration at Jennifer Wetzler blog Why Is A Pipette Used To Measure Sodium Hydroxide In association with nuffield foundation. Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. One type of titration uses a neutralization reaction, in which an acid and a base react to. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.webassign.net
Techniques Why Is A Pipette Used To Measure Sodium Hydroxide Use microscale titration to complete an. The pipette allows the same volume of acid to be added. In equation 1, the acid is hcl (called. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. In association with nuffield foundation. Using a volumetric pipet, measure out 25.00 ml. Why Is A Pipette Used To Measure Sodium Hydroxide.
From microbenotes.com
Pipette Principle, Parts, Types, Procedure, Uses, Examples Why Is A Pipette Used To Measure Sodium Hydroxide Explain why a pipette is used to measure the acid, rather than a measuring cylinder. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. Use microscale titration to complete an. Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. In equation. Why Is A Pipette Used To Measure Sodium Hydroxide.
From studylib.net
Standardizing a Sodium Hydroxide (NaOH) Solution Why Is A Pipette Used To Measure Sodium Hydroxide In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the. In association with nuffield foundation. The pipette allows the same volume of acid to be added. Explain why a pipette is used to measure the alkali,. Why Is A Pipette Used To Measure Sodium Hydroxide.
From microbenotes.com
Pipette Principle, Parts, Types, Procedure, Uses, Examples Why Is A Pipette Used To Measure Sodium Hydroxide Using a volumetric pipet, measure out 25.00 ml of the potassium hydrogen phthlate solution and put this in a 250 ml erlenmeyer flask. The pipette allows the same volume of acid to be added. In association with nuffield foundation. Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. One type of titration uses a. Why Is A Pipette Used To Measure Sodium Hydroxide.
From openlab.citytech.cuny.edu
Pipetting Basics Biology OER Why Is A Pipette Used To Measure Sodium Hydroxide Use microscale titration to complete an. In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt. Why Is A Pipette Used To Measure Sodium Hydroxide.
From www.alamy.com
microscale titration using a plastic pipette and a small vial. Mixing Why Is A Pipette Used To Measure Sodium Hydroxide In equation 1, the acid is hcl (called. Explain why a pipette is used to measure the acid, rather than a measuring cylinder. In association with nuffield foundation. Explain why a pipette is used to measure the alkali, rather than a measuring cylinder. Use microscale titration to complete an. The pipette allows the same volume of acid to be added.. Why Is A Pipette Used To Measure Sodium Hydroxide.