End Point In Redox Titration . Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while adding the. The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. A color change in the solution typically detects the endpoint. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. The endpoint of this titration is detected with the help of a starch indicator. Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic.
from www.slideserve.com
Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. A color change in the solution typically detects the endpoint. Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while adding the. The endpoint of this titration is detected with the help of a starch indicator. The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with.
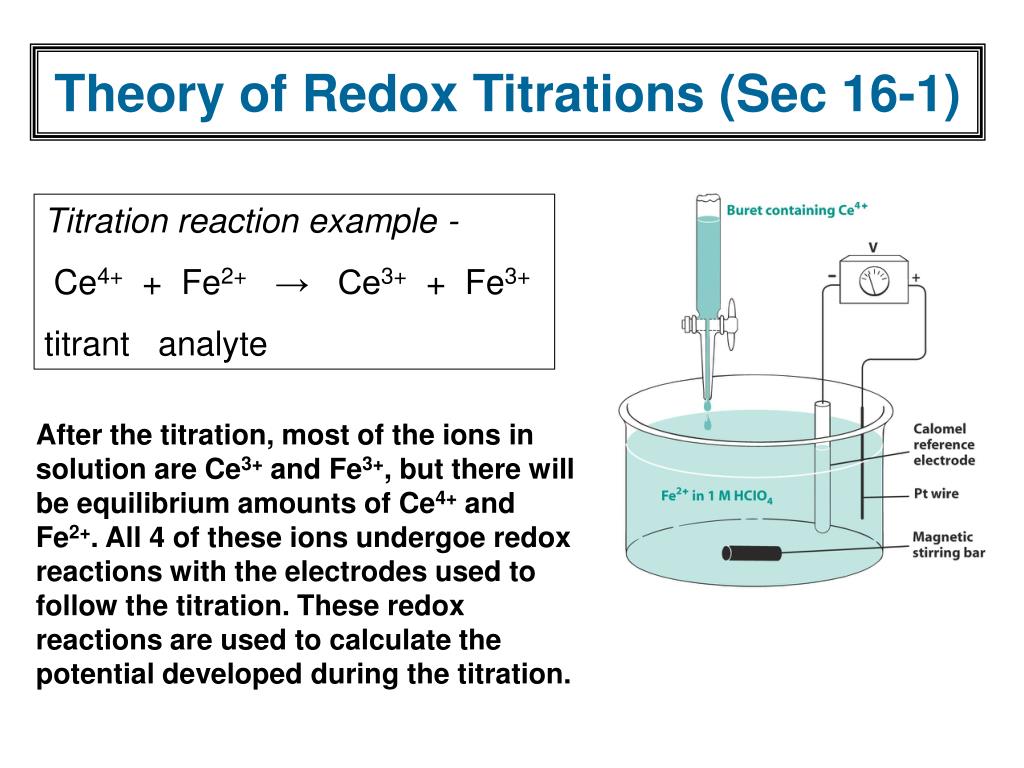
PPT Ch 16 Redox Titrations PowerPoint Presentation, free download
End Point In Redox Titration Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. A color change in the solution typically detects the endpoint. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while adding the. Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. The endpoint of this titration is detected with the help of a starch indicator. The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with.
From slideplayer.com
Unit 12 (Chp 20) Electrochemistry (E, ∆G, K) ppt download End Point In Redox Titration The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. The endpoint of this titration is detected with the help of a starch indicator. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage. End Point In Redox Titration.
From philschatz.com
AcidBase Titrations · Chemistry End Point In Redox Titration A color change in the solution typically detects the endpoint. The endpoint of this titration is detected with the help of a starch indicator. Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. The end point of a redox titration can be determined by either an electrode via measuring. End Point In Redox Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts End Point In Redox Titration Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. A color change in the solution typically detects the endpoint. The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. Consider a. End Point In Redox Titration.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 End Point In Redox Titration The endpoint of this titration is detected with the help of a starch indicator. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. Use. End Point In Redox Titration.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts End Point In Redox Titration A color change in the solution typically detects the endpoint. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while adding the. Use. End Point In Redox Titration.
From www.differencebetween.com
Difference Between AcidBase Titration and Redox Titration Compare End Point In Redox Titration Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. Consider a solution containing iron (ii) ions. End Point In Redox Titration.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts End Point In Redox Titration Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. The endpoint of this titration is detected with the help of a starch indicator. The end point of a redox titration can be detected by indicators, which change colour when all the. End Point In Redox Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre End Point In Redox Titration The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in. End Point In Redox Titration.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 End Point In Redox Titration A color change in the solution typically detects the endpoint. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element. End Point In Redox Titration.
From dxoqehvfn.blob.core.windows.net
End Point Of Redox Titration at Lester Klein blog End Point In Redox Titration A color change in the solution typically detects the endpoint. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while adding the. The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. The end point of a redox. End Point In Redox Titration.
From www.brainkart.com
Redox Titration Curves End Point In Redox Titration A color change in the solution typically detects the endpoint. Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. Another method for locating a redox titration’s end point is. End Point In Redox Titration.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 End Point In Redox Titration Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. The endpoint of this titration is detected with the help of a starch indicator. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. Use. End Point In Redox Titration.
From exykwxxst.blob.core.windows.net
Finding Titration End Point at John Graves blog End Point In Redox Titration Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. The end point of a redox titration can be detected by indicators,. End Point In Redox Titration.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 End Point In Redox Titration The endpoint of this titration is detected with the help of a starch indicator. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential. End Point In Redox Titration.
From slidetodoc.com
Redox Titrations Introduction 1 Redox Titration Based on End Point In Redox Titration A color change in the solution typically detects the endpoint. The endpoint of this titration is detected with the help of a starch indicator. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. The end point of a redox titration can be determined by. End Point In Redox Titration.
From exonshhzb.blob.core.windows.net
End Point Detection In Permanganate Titration at Marlene Ball blog End Point In Redox Titration The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. The endpoint of this titration is detected with the help of a. End Point In Redox Titration.
From scienceinfo.com
Redox titration Types, Applications, Advantages, Disadvantages End Point In Redox Titration The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has. End Point In Redox Titration.
From dxoqehvfn.blob.core.windows.net
End Point Of Redox Titration at Lester Klein blog End Point In Redox Titration Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. The end point of a redox titration can be detected by indicators,. End Point In Redox Titration.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 End Point In Redox Titration Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. Another method for locating a redox titration’s end point is a potentiometric titration in which. End Point In Redox Titration.
From studylib.net
Redox Titrations Introduction 1.) Redox Titration End Point In Redox Titration The endpoint of this titration is detected with the help of a starch indicator. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while adding the. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or. End Point In Redox Titration.
From slidetodoc.com
Chapter 15 Redox Titrations 15 1 The Shape End Point In Redox Titration Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. The endpoint of this titration is detected with the help of a starch indicator. A color change in the solution typically detects the endpoint. The end point of a redox titration can be determined by either an electrode via measuring. End Point In Redox Titration.
From www.chemistrylearner.com
Redox Titration Definition, Examples, and Applications End Point In Redox Titration Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while adding the. A color change in the solution typically detects the endpoint. The endpoint of this titration is detected with the help of a starch indicator. The end point of a redox titration can be detected by indicators,. End Point In Redox Titration.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts End Point In Redox Titration Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while adding the. The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. Use our revision notes to understand how a redox titration in a level chemistry can be. End Point In Redox Titration.
From dxoqehvfn.blob.core.windows.net
End Point Of Redox Titration at Lester Klein blog End Point In Redox Titration The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. A color change in the solution typically detects the endpoint. The endpoint of this titration is detected with the help. End Point In Redox Titration.
From dxoaiisdq.blob.core.windows.net
Titration Explained Simply at Marlene Barron blog End Point In Redox Titration The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. A color change in the solution typically detects the endpoint. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. The endpoint of this titration. End Point In Redox Titration.
From theedge.com.hk
Chemistry How To Titration The Edge End Point In Redox Titration Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while adding the. Use our revision notes to understand how a redox titration in a level chemistry can be. End Point In Redox Titration.
From www.slideserve.com
PPT Ch 16 Redox Titrations PowerPoint Presentation, free download End Point In Redox Titration Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. A color change in the solution typically detects the endpoint. The endpoint of this titration is detected with the help of a starch indicator. Another method for locating a redox titration’s end point is a potentiometric titration in which we. End Point In Redox Titration.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 End Point In Redox Titration The endpoint of this titration is detected with the help of a starch indicator. Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. Use. End Point In Redox Titration.
From dxoqehvfn.blob.core.windows.net
End Point Of Redox Titration at Lester Klein blog End Point In Redox Titration The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. Another method for locating a redox titration’s end point is a potentiometric. End Point In Redox Titration.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry End Point In Redox Titration The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while adding the. The endpoint of this titration is detected with the help of. End Point In Redox Titration.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 End Point In Redox Titration The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. Another method for locating a redox titration’s end point is a potentiometric titration in which. End Point In Redox Titration.
From www.brainkart.com
Selecting and Evaluating the End Point Titrations Based on Redox End Point In Redox Titration A color change in the solution typically detects the endpoint. The endpoint of this titration is detected with the help of a starch indicator. The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. Use our revision notes to understand how a redox titration in a level chemistry can be. End Point In Redox Titration.
From www.alamy.com
Potassium permanganate titration. At the start of a redox titration End Point In Redox Titration The endpoint of this titration is detected with the help of a starch indicator. The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. A color change in the solution typically detects the endpoint. Use our revision notes to understand how a redox titration in a level chemistry can be. End Point In Redox Titration.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download End Point In Redox Titration Consider a solution containing iron (ii) ions titrated with potassium dichromate (k 2 cr 2 o 7) solution under acidic. The end point of a redox titration can be detected by indicators, which change colour when all the analyte has reacted, or by using a. Another method for locating a redox titration’s end point is a potentiometric titration in which. End Point In Redox Titration.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation ID3080571 End Point In Redox Titration The end point of a redox titration can be determined by either an electrode via measuring the electrochemical potential with. A color change in the solution typically detects the endpoint. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. Consider a. End Point In Redox Titration.