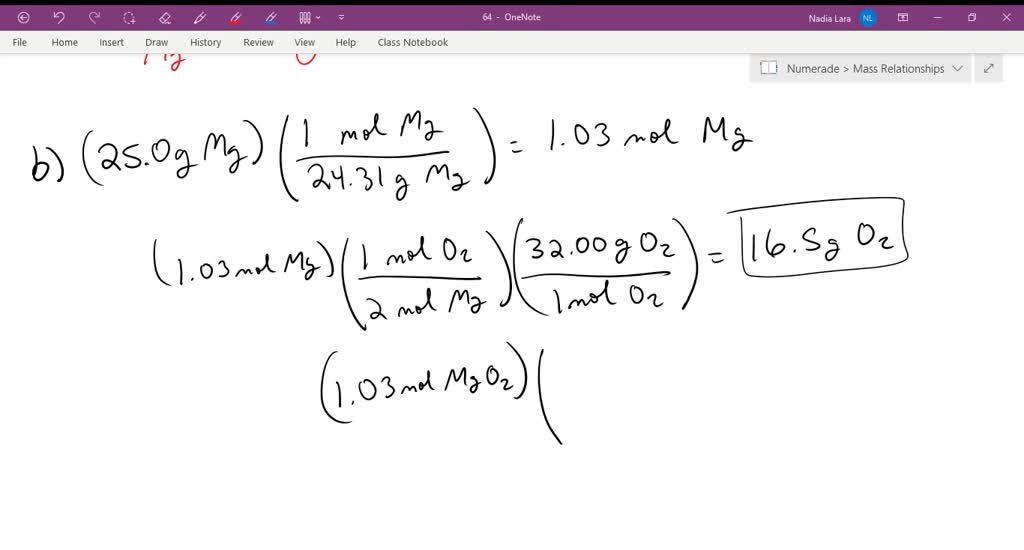
Magnesium Oxide Yield . Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. The balanced equation for the reaction is: Then we will use the percent yield equation to determine the percent yield of magnesium oxide. Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. The decomposition of magnesium carbonate forms 15 grams of magnesium oxide in an experiment. Magnesium reacts with oxygen as shown in the equation below: Mgco 3 → mgo + co 2 the calculation is simple if you know the actual and theoretical yields. All you need to do is plug the values into the formula: Complete each section of this report and. The theoretical yield is 19 grams. What is the percent yield of magnesium oxide?
from solvedlib.com
Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Magnesium reacts with oxygen as shown in the equation below: Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% The balanced equation for the reaction is: Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. What is the percent yield of magnesium oxide? The theoretical yield is 19 grams. All you need to do is plug the values into the formula: Then we will use the percent yield equation to determine the percent yield of magnesium oxide. The decomposition of magnesium carbonate forms 15 grams of magnesium oxide in an experiment.
Conversions of Amounts in MOLES to MASS Your Turn 2… SolvedLib
Magnesium Oxide Yield Complete each section of this report and. All you need to do is plug the values into the formula: Then we will use the percent yield equation to determine the percent yield of magnesium oxide. Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. The balanced equation for the reaction is: What is the percent yield of magnesium oxide? The decomposition of magnesium carbonate forms 15 grams of magnesium oxide in an experiment. Mgco 3 → mgo + co 2 the calculation is simple if you know the actual and theoretical yields. The theoretical yield is 19 grams. Complete each section of this report and. Magnesium reacts with oxygen as shown in the equation below: Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and.
From www.numerade.com
SOLVED "Find limiting reactant and calculate thc theoretical yield of Magnesium Oxide Yield Magnesium reacts with oxygen as shown in the equation below: Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. All you need. Magnesium Oxide Yield.
From www.numerade.com
A 0.100 g sample of magnesium, when combined with oxygen, yields 0.166 Magnesium Oxide Yield The decomposition of magnesium carbonate forms 15 grams of magnesium oxide in an experiment. The theoretical yield is 19 grams. Then we will use the percent yield equation to determine the percent yield of magnesium oxide. What is the percent yield of magnesium oxide? Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g /. Magnesium Oxide Yield.
From www.youtube.com
Chemistry Unit 2 The empirical formula and percentage yield of Magnesium Oxide Yield Mgco 3 → mgo + co 2 the calculation is simple if you know the actual and theoretical yields. What is the percent yield of magnesium oxide? All you need to do is plug the values into the formula: Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent. Magnesium Oxide Yield.
From www.numerade.com
SOLVEDMagnesium oxide can be made by heating magnesium metal in the Magnesium Oxide Yield Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Then we will use the percent yield equation to determine the percent yield of magnesium oxide. Magnesium reacts with oxygen as shown in the equation below: Mgco 3 → mgo + co 2 the calculation is simple if you know the actual and theoretical yields. Complete. Magnesium Oxide Yield.
From www.numerade.com
SOLVED 'Experiment 8 QUANTITIES IN CHEMICAL REACTIONS PreLab Magnesium Oxide Yield Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% The balanced equation for the reaction is: Magnesium reacts with oxygen as shown in the equation below: Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Then we will use the. Magnesium Oxide Yield.
From www.bartleby.com
Answered (ii) Magnesium reacts with oxygen to… bartleby Magnesium Oxide Yield The balanced equation for the reaction is: Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Magnesium reacts with oxygen as shown in the equation below: Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Percent yield = actual yield /. Magnesium Oxide Yield.
From childhealthpolicy.vumc.org
Magnesium oxide production lab. Lab Report 4 The Synthesis of Magnesium Oxide Yield The balanced equation for the reaction is: Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% Complete each section of this report and. Magnesium reacts with oxygen as shown in the equation below: Magnesium oxide can be made by heating magnesium metal in the presence. Magnesium Oxide Yield.
From www.numerade.com
Magnesium burns in air with a dazzling brilliance to produce magnesium Magnesium Oxide Yield Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. The decomposition of magnesium carbonate forms 15 grams of magnesium oxide in an experiment. Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield =. Magnesium Oxide Yield.
From www.numerade.com
SOLVED Magnesium burns in air with dazzling brilliance to produce Magnesium Oxide Yield Mgco 3 → mgo + co 2 the calculation is simple if you know the actual and theoretical yields. Complete each section of this report and. Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations. Magnesium Oxide Yield.
From www.numerade.com
SOLVEDMagnesium oxide can be made by heating magnesium metal in the Magnesium Oxide Yield All you need to do is plug the values into the formula: The balanced equation for the reaction is: Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. The decomposition of magnesium. Magnesium Oxide Yield.
From www.youtube.com
Percentage yield of magnesium oxide YouTube Magnesium Oxide Yield The balanced equation for the reaction is: Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Complete each section of this report and. The theoretical yield is 19 grams. Mgco 3 →. Magnesium Oxide Yield.
From www.chegg.com
Solved See page 306 04 Question (4 points) A 0.266 g piece Chegg Magnesium Oxide Yield What is the percent yield of magnesium oxide? All you need to do is plug the values into the formula: Then we will use the percent yield equation to determine the percent yield of magnesium oxide. Complete each section of this report and. Magnesium reacts with oxygen as shown in the equation below: Magnesium oxide, also known as magnesia, is. Magnesium Oxide Yield.
From studylib.net
Magnesium Oxide Percent Yield Lab Magnesium Oxide Yield The balanced equation for the reaction is: The theoretical yield is 19 grams. Complete each section of this report and. Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Magnesium reacts with oxygen as shown in the equation below: Mgco 3 → mgo + co 2 the calculation is simple if you know the actual. Magnesium Oxide Yield.
From www.studocu.com
5.065.07 percent yield lab Title Percent Yield of Magnesium Oxide Magnesium Oxide Yield Then we will use the percent yield equation to determine the percent yield of magnesium oxide. The decomposition of magnesium carbonate forms 15 grams of magnesium oxide in an experiment. Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. The balanced equation for the reaction is: The theoretical yield is 19 grams. Magnesium reacts with. Magnesium Oxide Yield.
From www.crystran.co.uk
Magnesium Oxide MgO Magnesium Oxide Yield Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Magnesium oxide can be made by heating magnesium metal in the presence of. Magnesium Oxide Yield.
From schoolworkhelper.net
Magnesium Oxide Percent Yield Lab Report SchoolWorkHelper Magnesium Oxide Yield Complete each section of this report and. The theoretical yield is 19 grams. The balanced equation for the reaction is: Then we will use the percent yield equation to determine the percent yield of magnesium oxide. The decomposition of magnesium carbonate forms 15 grams of magnesium oxide in an experiment. What is the percent yield of magnesium oxide? Magnesium reacts. Magnesium Oxide Yield.
From www.coursehero.com
[Solved] Magnesium oxide and ammonium bromide react to produce ammonia Magnesium Oxide Yield Complete each section of this report and. Mgco 3 → mgo + co 2 the calculation is simple if you know the actual and theoretical yields. The decomposition of magnesium carbonate forms 15 grams of magnesium oxide in an experiment. Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100%. Magnesium Oxide Yield.
From healthjade.net
Magnesium Oxide Uses, Benefits, Dosage, Side Effects Magnesium Oxide Yield The decomposition of magnesium carbonate forms 15 grams of magnesium oxide in an experiment. Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% Then we will use the percent yield equation to determine the percent yield of magnesium oxide. Mgco 3 → mgo + co. Magnesium Oxide Yield.
From medium.com
Magnesium Oxide (MgO) Nanoparticles — Properties and Applications Magnesium Oxide Yield Magnesium reacts with oxygen as shown in the equation below: What is the percent yield of magnesium oxide? Then we will use the percent yield equation to determine the percent yield of magnesium oxide. Complete each section of this report and. Mgco 3 → mgo + co 2 the calculation is simple if you know the actual and theoretical yields.. Magnesium Oxide Yield.
From www.nagwa.com
Question Video Calculating the Mass of Oxygen Required to React with a Magnesium Oxide Yield The decomposition of magnesium carbonate forms 15 grams of magnesium oxide in an experiment. Magnesium reacts with oxygen as shown in the equation below: Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Then we will use the percent yield equation to determine the percent yield of magnesium oxide. Mgco 3 → mgo + co. Magnesium Oxide Yield.
From www.slideserve.com
PPT 1. Magnesium metal combines with oxygen gas to produce magnesium Magnesium Oxide Yield Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Complete each section of this report and. The theoretical yield is 19 grams. The decomposition of magnesium carbonate forms 15 grams of magnesium oxide in an experiment. Magnesium oxide can be made by heating magnesium metal in. Magnesium Oxide Yield.
From www.youtube.com
Finding Percentage Yield of Magnesium Oxide Prac YouTube Magnesium Oxide Yield Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. What is the percent yield of magnesium oxide? Complete each section of this report and. Magnesium oxide, also known as magnesia, is. Magnesium Oxide Yield.
From www.chegg.com
Solved LAB Empirical formula, Yield of Magnesium Oxide Magnesium Oxide Yield All you need to do is plug the values into the formula: Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. The decomposition of magnesium carbonate forms 15 grams of magnesium oxide in an experiment. What is the percent yield of magnesium oxide? Complete each section of this report and. Magnesium reacts with oxygen as. Magnesium Oxide Yield.
From www.numerade.com
SOLVED Experiment 7 Empirical Formula Objectives Determine the Magnesium Oxide Yield Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Then we will use the percent yield equation to determine the percent yield of magnesium oxide. The theoretical yield is 19 grams. Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Complete. Magnesium Oxide Yield.
From childhealthpolicy.vumc.org
Magnesium oxide production lab. Lab Report 4 The Synthesis of Magnesium Oxide Yield Mgco 3 → mgo + co 2 the calculation is simple if you know the actual and theoretical yields. The balanced equation for the reaction is: Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% Magnesium reacts with oxygen as shown in the equation below:. Magnesium Oxide Yield.
From www.marketresearchfuture.com
Magnesium Oxide Market Size, Share & Report, 2032 Magnesium Oxide Yield The theoretical yield is 19 grams. All you need to do is plug the values into the formula: Mgco 3 → mgo + co 2 the calculation is simple if you know the actual and theoretical yields. The balanced equation for the reaction is: Then we will use the percent yield equation to determine the percent yield of magnesium oxide.. Magnesium Oxide Yield.
From www.youtube.com
How to Balance MgO + H2O = Mg(OH)2 (Magnesium Oxide plus Water) YouTube Magnesium Oxide Yield The balanced equation for the reaction is: The theoretical yield is 19 grams. Magnesium reacts with oxygen as shown in the equation below: All you need to do is plug the values into the formula: Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Then we. Magnesium Oxide Yield.
From www.solutionspile.com
[Solved] A ( 0.236 mathrm{g} ) piece of solid magnesiu Magnesium Oxide Yield All you need to do is plug the values into the formula: What is the percent yield of magnesium oxide? Complete each section of this report and. The theoretical yield is 19 grams. Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Then we will use the percent yield equation to determine the percent yield. Magnesium Oxide Yield.
From solvedlib.com
Conversions of Amounts in MOLES to MASS Your Turn 2… SolvedLib Magnesium Oxide Yield The theoretical yield is 19 grams. Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% Then we will use the percent yield equation to determine the percent yield of magnesium oxide. Magnesium reacts with oxygen as shown in the equation below: All you need to. Magnesium Oxide Yield.
From www.numerade.com
SOLVED YIELD OF A CHEMICAL REACTION EXPERIMENT NOTES this experiment Magnesium Oxide Yield Magnesium reacts with oxygen as shown in the equation below: What is the percent yield of magnesium oxide? Then we will use the percent yield equation to determine the percent yield of magnesium oxide. Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Percent yield = actual yield / theoretical yield x 100% percent yield. Magnesium Oxide Yield.
From www.youtube.com
How to Write the Formula for MgO (Magnesium oxide) YouTube Magnesium Oxide Yield Mgco 3 → mgo + co 2 the calculation is simple if you know the actual and theoretical yields. Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% The balanced equation for the reaction is: Magnesium reacts with oxygen as shown in the equation below:. Magnesium Oxide Yield.
From www.numerade.com
Magnesium oxide can be made by heating magnesium metal in the presence Magnesium Oxide Yield The decomposition of magnesium carbonate forms 15 grams of magnesium oxide in an experiment. All you need to do is plug the values into the formula: Mgco 3 → mgo + co 2 the calculation is simple if you know the actual and theoretical yields. Magnesium reacts with oxygen as shown in the equation below: Magnesium oxide can be made. Magnesium Oxide Yield.
From www.youtube.com
Formation of Magnesium Oxide (MgO) Chemical Bonding Atomic Magnesium Oxide Yield Mgco 3 → mgo + co 2 the calculation is simple if you know the actual and theoretical yields. What is the percent yield of magnesium oxide? The theoretical yield is 19 grams. Magnesium reacts with oxygen as shown in the equation below: Complete each section of this report and. Magnesium oxide, also known as magnesia, is a classic example. Magnesium Oxide Yield.
From www.researchgate.net
Magnesium Oxide Recovery according to the experimental statistical Magnesium Oxide Yield Mgco 3 → mgo + co 2 the calculation is simple if you know the actual and theoretical yields. The balanced equation for the reaction is: Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% The decomposition of magnesium carbonate forms 15 grams of magnesium. Magnesium Oxide Yield.
From www.scribd.com
Synthesis of Magnesium Oxide and Calculation of Percentage Yield PDF Magnesium Oxide Yield What is the percent yield of magnesium oxide? The balanced equation for the reaction is: Magnesium reacts with oxygen as shown in the equation below: Complete each section of this report and. All you need to do is plug the values into the formula: Mgco 3 → mgo + co 2 the calculation is simple if you know the actual. Magnesium Oxide Yield.