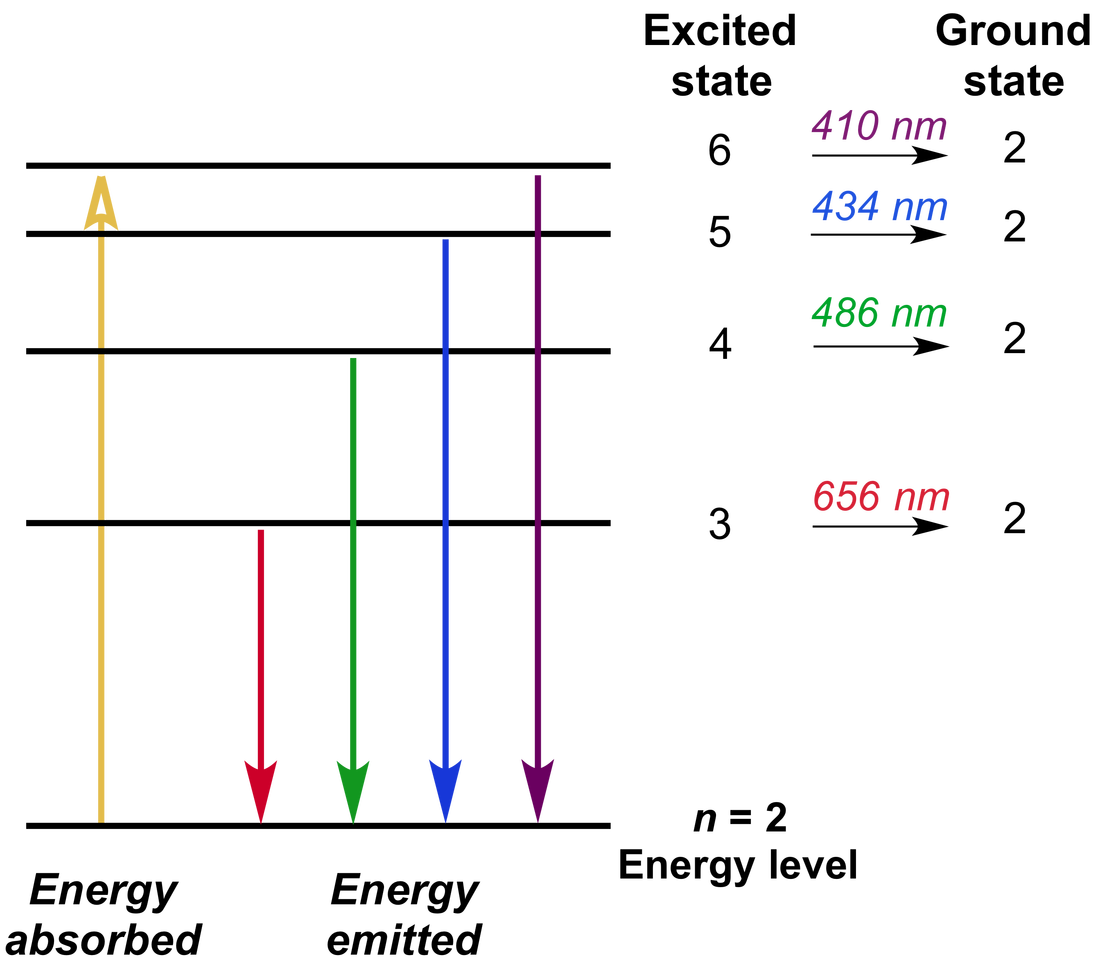
Spectroscopy Ground State . Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: For an atom in its ground state, the. The spectra of transition metal complexes is not as simple as it appears from just the splitting of d.
from chemistrypuns-periodically.weebly.com
The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. For an atom in its ground state, the. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which.
Chemistry Electron Emission Spectrum
Spectroscopy Ground State The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. For an atom in its ground state, the. the allocation electrons among degenerate orbitals can be formalized by hund’s rule:
From www.researchgate.net
FIG. SI4 Excited state spectroscopy. (a) Energy level diagram of the... Download Scientific Spectroscopy Ground State For an atom in its ground state, the. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: The spectra of transition metal complexes is not as simple as it appears from just the. Spectroscopy Ground State.
From www.slideserve.com
PPT Laser Spectroscopy Ground State Properties of Exotic Nuclei PowerPoint Presentation ID Spectroscopy Ground State This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. Basic properties of atoms.possible energy state (called the ground state) can. Spectroscopy Ground State.
From www.researchgate.net
Timeresolved ground state bleach spectrum isolated from transient... Download Scientific Diagram Spectroscopy Ground State Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. For an atom in its ground state, the. understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. the electrons in an atom tend to be arranged. Spectroscopy Ground State.
From www.researchgate.net
Upper panel Exact energy spectra (ground state and six lowest... Download Scientific Diagram Spectroscopy Ground State The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. For an atom. Spectroscopy Ground State.
From dokumen.tips
(PDF) Infrared Spectroscopy ofMolecular Supernova RemnantsISO SWS spectra of the groundstate Spectroscopy Ground State This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. For an atom in its ground state, the. the allocation electrons among degenerate orbitals can be formalized by hund’s. Spectroscopy Ground State.
From www.researchgate.net
(PDF) Twophoton laser induced fluorescence spectroscopy a powerful diagnostic tool to monitor Spectroscopy Ground State understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. For an atom in its ground state, the. The spectra of transition metal complexes is not. Spectroscopy Ground State.
From www.researchgate.net
(PDF) Groundstate interpretation of xray emission spectroscopy on adsorbates CO adsorbed on Spectroscopy Ground State the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. For an atom in its ground state, the. the allocation electrons. Spectroscopy Ground State.
From socratic.org
What is Groundstate and Excited state of an atom?Thanks.. Socratic Spectroscopy Ground State understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. For an atom in its ground state, the. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. The spectra of transition metal complexes is not as simple. Spectroscopy Ground State.
From www.researchgate.net
(PDF) Study of the rovibrational levels of ground state excited hydrogen molecules by laser Spectroscopy Ground State understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. For an atom in its ground state, the. . Spectroscopy Ground State.
From chem.libretexts.org
10.6 Photoluminescence Spectroscopy Chemistry LibreTexts Spectroscopy Ground State the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only. Spectroscopy Ground State.
From www.researchgate.net
Basic principle of Raman spectroscopy (a) v0 represents the vibrational... Download Scientific Spectroscopy Ground State understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. The spectra of transition metal complexes is not as simple as it appears from just the splitting of. Spectroscopy Ground State.
From www.researchgate.net
(PDF) Broadband EPR spectroscopy of the ground electron state of the Fe4+ impurity ion in amethyst. Spectroscopy Ground State The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from. Spectroscopy Ground State.
From www.researchgate.net
Experimentally obtained LAC spectra for the ground state (GS) and the... Download Scientific Spectroscopy Ground State For an atom in its ground state, the. understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: Basic. Spectroscopy Ground State.
From webmis.highland.cc.il.us
Atomic Spectra and Models of the Atom Spectroscopy Ground State This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. The spectra of transition metal complexes is not as simple as. Spectroscopy Ground State.
From www.researchgate.net
(a) Dependence of the formation dynamics of groundstate bleach (PB1)... Download Scientific Spectroscopy Ground State Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: For an atom in its. Spectroscopy Ground State.
From www.researchgate.net
Ground state electronic absorption spectra of [Fe(bpy) 3 ](PF 6 ) 2... Download Scientific Diagram Spectroscopy Ground State This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. For an atom in its ground state, the. understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: Basic properties of. Spectroscopy Ground State.
From www.researchgate.net
Broadband EPR Spectroscopy of the Ground Electron State of the Fe Impurity Ion in Amethyst Spectroscopy Ground State For an atom in its ground state, the. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. the allocation electrons among degenerate orbitals can be formalized. Spectroscopy Ground State.
From www.researchgate.net
(PDF) Direct observation of groundstate lactamlactim tautomerization using temperaturejump Spectroscopy Ground State The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. the electrons in an atom tend to be. Spectroscopy Ground State.
From dxoyseznp.blob.core.windows.net
Ground State Terms at Larry Flannery blog Spectroscopy Ground State understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. For an atom in its ground state, the. The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: Basic. Spectroscopy Ground State.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Spectroscopy Ground State the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. For an atom in its ground state, the. The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. understanding ground state is essential for grasping. Spectroscopy Ground State.
From classnotes.org.in
Hydrogen spectrum Chemistry, Class 11, Structure Of Atom Spectroscopy Ground State The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: For an atom. Spectroscopy Ground State.
From www.researchgate.net
(PDF) Collinear laser spectroscopy on the ground state and an excited state in neutral ^{55}Mn Spectroscopy Ground State This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: For an atom in its ground state, the. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added. Spectroscopy Ground State.
From www.studocu.com
Molecular Spectroscopy notes 1 MOLECULAR SPECTROSCOPY Apart from the ground state a molecule Spectroscopy Ground State This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. For an atom in its ground state, the. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: the electrons in an atom tend to be arranged in such a way that the energy of the atom is as. Spectroscopy Ground State.
From byjus.com
Explain Hund's rule and spectroscopic term of the ground state of an atom? Spectroscopy Ground State The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. For an atom in its ground state, the. the electrons in an atom tend to be arranged in such a way. Spectroscopy Ground State.
From www.researchgate.net
(PDF) First trapassisted decay spectroscopy of the 81Ge ground state Spectroscopy Ground State For an atom in its ground state, the. understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. Basic properties of atoms.possible energy state (called the ground state) can be excited to. Spectroscopy Ground State.
From www.toppr.com
The emission spectra is observed by the consequence of transition of electron from higher energy Spectroscopy Ground State the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: For an atom in its. Spectroscopy Ground State.
From www.researchgate.net
a) Upper trace FTIR steadystate (ground state, S 0 ) spectra of 3 at... Download Scientific Spectroscopy Ground State the allocation electrons among degenerate orbitals can be formalized by hund’s rule: understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. Basic properties of. Spectroscopy Ground State.
From www.researchgate.net
(PDF) Fourier transform spectroscopy of the COstretching band of C13 methanol in the torsional Spectroscopy Ground State the allocation electrons among degenerate orbitals can be formalized by hund’s rule: This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. Basic properties of atoms.possible energy state (called the ground state) can. Spectroscopy Ground State.
From jascoinc.com
Fluorescence Spectroscopy JASCO Spectroscopy Ground State For an atom in its ground state, the. the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. the allocation electrons. Spectroscopy Ground State.
From www.researchgate.net
(a) PKED in He(I) photoelectron spectroscopy of groundstate pyrazine... Download Scientific Spectroscopy Ground State For an atom in its ground state, the. the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. Basic properties of atoms.possible energy state (called the ground state). Spectroscopy Ground State.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Spectroscopy Ground State the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: For an atom in its ground state, the. This module is designed to introduce the basic concepts of spectroscopy and to provide. Spectroscopy Ground State.
From www.researchgate.net
(PDF) Fourier transform spectroscopy of BaO New groundstate constants from the A 1Σ+X 1Σ Spectroscopy Ground State For an atom in its ground state, the. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of. The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher. Spectroscopy Ground State.
From chemistnotes.com
Atomic Fluorescence Spectroscopy Principle, Instrumentation, and 7 Reliable Applications Spectroscopy Ground State the allocation electrons among degenerate orbitals can be formalized by hund’s rule: Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. understanding ground state is. Spectroscopy Ground State.
From www.researchgate.net
(PDF) Application of nearinfrared luminescence spectroscopy to vanadium(III) complexes Spectroscopy Ground State The spectra of transition metal complexes is not as simple as it appears from just the splitting of d. Basic properties of atoms.possible energy state (called the ground state) can be excited to a higher state only if energy is added by. the electrons in an atom tend to be arranged in such a way that the energy of. Spectroscopy Ground State.
From www.sliderbase.com
Hybridization of Orbitals Presentation Chemistry Spectroscopy Ground State understanding ground state is essential for grasping atomic behavior in spectroscopy because it sets the baseline from which. the allocation electrons among degenerate orbitals can be formalized by hund’s rule: the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. For an atom. Spectroscopy Ground State.