Igcse Titration . They can determine exactly how much alkali is needed to neutralise. Titrations are a method of analysing the concentration of solutions. Find examples of titrations with acids, alkalis and. Learn how to carry out a titration. The diagram shows the titration. Download pdf notes, watch video preview and access paid. Titrations are a method of analysing the concentration of solutions. Titration is used to find out precisely how much acid neutralises a certain volume of alkali (or vice versa). They can determine exactly how much alkali is. Learn how to perform titrations using a burette, indicator and concordant results. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to. Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation.
from www.youtube.com
Find examples of titrations with acids, alkalis and. Learn how to perform titrations using a burette, indicator and concordant results. They can determine exactly how much alkali is. Titrations are a method of analysing the concentration of solutions. Learn how to carry out a titration. The diagram shows the titration. Titrations are a method of analysing the concentration of solutions. They can determine exactly how much alkali is needed to neutralise. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. Titration is used to find out precisely how much acid neutralises a certain volume of alkali (or vice versa).
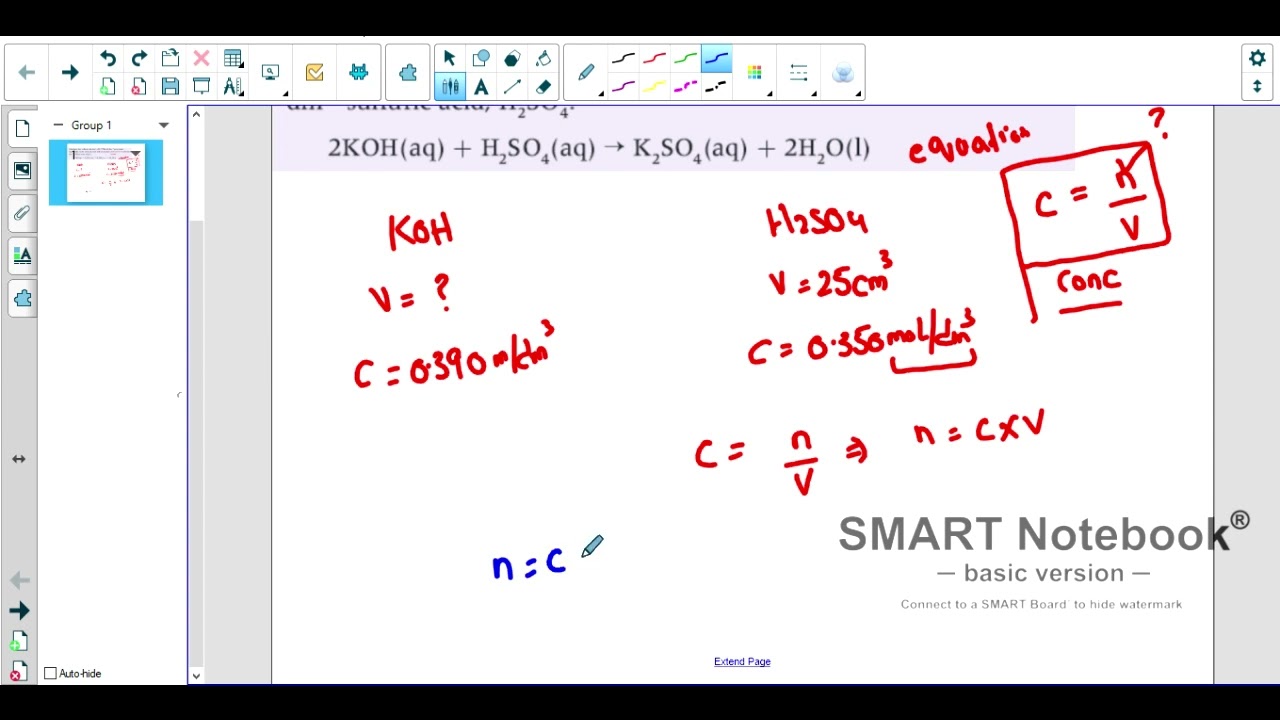
IB, IGCSE, FSC Acid Base Titration, Concentration with example
Igcse Titration Titrations are a method of analysing the concentration of solutions. Download pdf notes, watch video preview and access paid. They can determine exactly how much alkali is needed to neutralise. The diagram shows the titration. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. Titrations are a method of analysing the concentration of solutions. Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation. Find examples of titrations with acids, alkalis and. Titrations are a method of analysing the concentration of solutions. Titration is used to find out precisely how much acid neutralises a certain volume of alkali (or vice versa). Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to. Learn how to carry out a titration. Learn how to perform titrations using a burette, indicator and concordant results. They can determine exactly how much alkali is.
From www.youtube.com
Titration Calculation with IGCSE Chemistry Past Year Paper Questions Igcse Titration Titration is used to find out precisely how much acid neutralises a certain volume of alkali (or vice versa). Learn how to perform titrations using a burette, indicator and concordant results. Download pdf notes, watch video preview and access paid. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. They can. Igcse Titration.
From www.studypool.com
SOLUTION Igcse chem topic questions titration Studypool Igcse Titration Learn how to carry out a titration. Download pdf notes, watch video preview and access paid. Titrations are a method of analysing the concentration of solutions. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. Learn how to perform titrations using a burette, indicator and concordant results. They can determine exactly. Igcse Titration.
From www.youtube.com
IGCSE Titration Preparation and Testing of insoluble Salts YouTube Igcse Titration They can determine exactly how much alkali is. Download pdf notes, watch video preview and access paid. Learn how to perform titrations using a burette, indicator and concordant results. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. Find examples of titrations with acids, alkalis and. Titrations are carried out to. Igcse Titration.
From www.studypool.com
SOLUTION Igcse chem topic questions titration Studypool Igcse Titration Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation. Learn how to perform titrations using a burette, indicator and concordant results. They can determine exactly how much alkali is. Find examples of titrations with acids, alkalis and. Download pdf notes, watch video preview and access paid. Learn how to carry out a titration.. Igcse Titration.
From www.studypool.com
SOLUTION Acid alkali and titration chemistry igcse edexcel notes 2 Igcse Titration They can determine exactly how much alkali is. Download pdf notes, watch video preview and access paid. They can determine exactly how much alkali is needed to neutralise. Find examples of titrations with acids, alkalis and. The diagram shows the titration. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. Titrations. Igcse Titration.
From www.tes.com
Edexcel IGCSE Chemistry Lesson 22 Titrations Teaching Resources Igcse Titration Download pdf notes, watch video preview and access paid. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. Titrations are a method of analysing the concentration of solutions. Titrations. Igcse Titration.
From www.youtube.com
GCSE, IGCSE, O, A Level Chemistry 5070, 0620, 6092, 9701 Practicals Igcse Titration They can determine exactly how much alkali is. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. The diagram shows the titration. Titrations are a method of analysing the concentration of solutions. They can determine exactly how much alkali is needed to neutralise. Titrations are a method of analysing the concentration. Igcse Titration.
From www.vrogue.co
Chemistry Notes Igcse Gcse Olevel Titration Igcse Gcs vrogue.co Igcse Titration The diagram shows the titration. Learn how to perform titrations using a burette, indicator and concordant results. Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation. Titrations are a method of analysing the concentration of solutions. They can determine exactly how much alkali is. Learn the definition, examples and properties of acids and. Igcse Titration.
From www.youtube.com
OL & IGCSE Chemistry Titration YouTube Igcse Titration Titrations are a method of analysing the concentration of solutions. Download pdf notes, watch video preview and access paid. They can determine exactly how much alkali is. Learn how to carry out a titration. Titration is used to find out precisely how much acid neutralises a certain volume of alkali (or vice versa). Find examples of titrations with acids, alkalis. Igcse Titration.
From resource.studiaacademy.com
edexcel_igcse_chemistry_topic16_acidsbasesandsaltpreparations_004 Igcse Titration Download pdf notes, watch video preview and access paid. The diagram shows the titration. Titrations are a method of analysing the concentration of solutions. Learn how to carry out a titration. Titration is used to find out precisely how much acid neutralises a certain volume of alkali (or vice versa). Titrations are carried out to calculate the concentration of an. Igcse Titration.
From www.studypool.com
SOLUTION Igcse chem topic questions titration Studypool Igcse Titration Titrations are a method of analysing the concentration of solutions. Learn how to carry out a titration. Titrations are a method of analysing the concentration of solutions. They can determine exactly how much alkali is needed to neutralise. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to.. Igcse Titration.
From www.youtube.com
GCSE, IGCSE, O Level Chemistry 5070, 0620, 6092 Redox titration Igcse Titration They can determine exactly how much alkali is needed to neutralise. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. Titrations are a method of analysing the concentration of solutions. Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation. The diagram shows the titration.. Igcse Titration.
From www.thinkswap.com
Stage 1 Titration Chemistry Year 11 SACE Thinkswap Igcse Titration Download pdf notes, watch video preview and access paid. Titrations are a method of analysing the concentration of solutions. They can determine exactly how much alkali is needed to neutralise. Learn how to carry out a titration. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to. Find. Igcse Titration.
From mungfali.com
Titration Lab Diagram Igcse Titration Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation. The diagram shows the titration. Titrations are a method of analysing the concentration of solutions. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to. Find examples of titrations with acids, alkalis. Igcse Titration.
From practical-science.com
How to Determine the Concentration of a Hydrochloric Acid Solution Igcse Titration Download pdf notes, watch video preview and access paid. They can determine exactly how much alkali is needed to neutralise. Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation. Learn how to perform titrations using a burette, indicator and concordant results. Titrations are a method of analysing the concentration of solutions. The diagram. Igcse Titration.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations* — the science sauce Igcse Titration Learn how to perform titrations using a burette, indicator and concordant results. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. The diagram shows the titration. Titrations are a method of analysing the concentration of solutions. Titrations are carried out to calculate the concentration of an acid or an alkali by. Igcse Titration.
From www.savemyexams.co.uk
AcidBase Titrations (12.1.3) CIE IGCSE Chemistry Revision Notes 2023 Igcse Titration Titrations are a method of analysing the concentration of solutions. Titrations are a method of analysing the concentration of solutions. Learn how to perform titrations using a burette, indicator and concordant results. Find examples of titrations with acids, alkalis and. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is. Igcse Titration.
From dokumen.tips
IGCSE Titration Practice Questions Igcse Titration They can determine exactly how much alkali is. Learn how to carry out a titration. The diagram shows the titration. Titrations are a method of analysing the concentration of solutions. They can determine exactly how much alkali is needed to neutralise. Download pdf notes, watch video preview and access paid. Learn the definition, examples and properties of acids and alkalis,. Igcse Titration.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Igcse Titration Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation. Download pdf notes, watch video preview and access paid. Titrations are a method of analysing the concentration of solutions. Titration is used to find out precisely how much acid neutralises a certain volume of alkali (or vice versa). Titrations are a method of analysing. Igcse Titration.
From www.scribd.com
IGCSE Titration Practice Questions Igcse Titration They can determine exactly how much alkali is. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to. Find examples of titrations with acids, alkalis and. Titration is used to find out precisely how much acid neutralises a certain volume of alkali (or vice versa). Titrations are a. Igcse Titration.
From www.scribd.com
Chemistry IGCSE Titration calculation PDF Igcse Titration The diagram shows the titration. Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation. Learn how to carry out a titration. Titrations are a method of analysing the concentration of solutions. They can determine exactly how much alkali is. Titrations are a method of analysing the concentration of solutions. Find examples of titrations. Igcse Titration.
From resource.studiaacademy.com
edexcel_igcse_chemistry_topic15_acidsalkalisandtitrations_004 Igcse Titration Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. The diagram shows the titration. They can determine exactly how much alkali is. Learn how to carry out a titration. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to.. Igcse Titration.
From www.youtube.com
iGCSE Chemistry Acid Base L8 Titration calculations YouTube Igcse Titration Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. Titration is used to find out precisely how much acid neutralises a certain volume of alkali (or vice versa). Learn how to perform titrations using a burette, indicator and concordant results. Titrations are a method of analysing the concentration of solutions. Learn. Igcse Titration.
From igcse-chemistry-edexcel.blogspot.com
IGCSE Chemistry 4.9 describe experiments to carry out acidalkali Igcse Titration Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to. Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation. Titrations are a method. Igcse Titration.
From www.studocu.com
IGCSE CHEMISTRY(620) GUIDE TO CALCULATIONS IN ACIDBASE TITRATIONS Igcse Titration Learn how to carry out a titration. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to. The diagram shows the titration. Titrations are a method of analysing the concentration of solutions. They can determine exactly how much alkali is needed to neutralise. Download pdf notes, watch video. Igcse Titration.
From www.tes.com
Edexcel IGCSE Chemistry Worksheet 22 Titrations Teaching Resources Igcse Titration Learn how to carry out a titration. Titrations are a method of analysing the concentration of solutions. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to. They can determine exactly how much alkali is. The diagram shows the titration. Titration is used to find out precisely how. Igcse Titration.
From www.savemyexams.com
AcidBase Titrations CIE IGCSE Chemistry Revision Notes 2023 Igcse Titration Titrations are a method of analysing the concentration of solutions. Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation. Find examples of titrations with acids, alkalis and. Titrations are a method of analysing the concentration of solutions. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration. Igcse Titration.
From www.scribd.com
Good Igcse Cie Question 4 PDF Titration Chemistry Igcse Titration Learn how to perform titrations using a burette, indicator and concordant results. Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation. Titrations are a method of analysing the concentration of solutions. They can determine exactly how much alkali is needed to neutralise. Learn how to carry out a titration. Learn the definition, examples. Igcse Titration.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 2.7.6 Prepare a Soluble Salt II翰林国际教育 Igcse Titration They can determine exactly how much alkali is. The diagram shows the titration. Titration is used to find out precisely how much acid neutralises a certain volume of alkali (or vice versa). Titrations are a method of analysing the concentration of solutions. Titrations are a method of analysing the concentration of solutions. Find examples of titrations with acids, alkalis and.. Igcse Titration.
From www.tes.com
Edexcel IGCSE Chemistry Lecture 22 Titrations Teaching Resources Igcse Titration They can determine exactly how much alkali is needed to neutralise. Find examples of titrations with acids, alkalis and. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to. Titrations. Igcse Titration.
From www.youtube.com
IGCSE Chemistry Titration and indicators method question YouTube Igcse Titration Download pdf notes, watch video preview and access paid. The diagram shows the titration. Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation. Titrations are a method of analysing the concentration of solutions. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. Titration is. Igcse Titration.
From www.youtube.com
ATP Titration Questions O level and IGCSE Chemistry Ahmed Igcse Titration Titration is a technique to measure the volumes of acid and alkali solutions required for neutralisation. Learn how to carry out a titration. Titrations are a method of analysing the concentration of solutions. They can determine exactly how much alkali is. Learn how to perform titrations using a burette, indicator and concordant results. They can determine exactly how much alkali. Igcse Titration.
From www.studypool.com
SOLUTION Igcse chem topic questions titration Studypool Igcse Titration Titrations are a method of analysing the concentration of solutions. Download pdf notes, watch video preview and access paid. They can determine exactly how much alkali is needed to neutralise. Learn the definition, examples and properties of acids and alkalis, and how to measure their concentration by titration. Learn how to carry out a titration. Titrations are a method of. Igcse Titration.
From www.youtube.com
IB, IGCSE, FSC Acid Base Titration, Concentration with example Igcse Titration Find examples of titrations with acids, alkalis and. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to. The diagram shows the titration. Titration is used to find out precisely how much acid neutralises a certain volume of alkali (or vice versa). Titration is a technique to measure. Igcse Titration.
From www.vrogue.co
Chemistry Notes Igcse Gcse Olevel Titration Igcse Gcs vrogue.co Igcse Titration Titrations are a method of analysing the concentration of solutions. They can determine exactly how much alkali is. Titrations are carried out to calculate the concentration of an acid or an alkali by determining how much acid is needed to. Download pdf notes, watch video preview and access paid. Titration is a technique to measure the volumes of acid and. Igcse Titration.