Laws About Energy Changes . Thermodynamics is the study of the relations between heat, work, temperature, and energy. It states that there is a. A car engine burns gasoline, converting the chemical energy. Laws of thermodynamics, four relations underlying thermodynamics, the branch of physics concerning heat, work, temperature,. Δ u = q − w. Energy changes from one form of energy into another form of energy. There is no known exception to this law—it is exact so far as we know. The laws of thermodynamics describe how the energy in a system. A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. The law of conservation of energy states that the total energy is constant in any process. The law is called the conservation of energy. Energy may change in form or be transferred from one.
from www.teachoo.com
There is no known exception to this law—it is exact so far as we know. The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. Energy changes from one form of energy into another form of energy. Laws of thermodynamics, four relations underlying thermodynamics, the branch of physics concerning heat, work, temperature,. The laws of thermodynamics describe how the energy in a system. Δ u = q − w. Thermodynamics is the study of the relations between heat, work, temperature, and energy. The law is called the conservation of energy. Energy may change in form or be transferred from one. A car engine burns gasoline, converting the chemical energy.
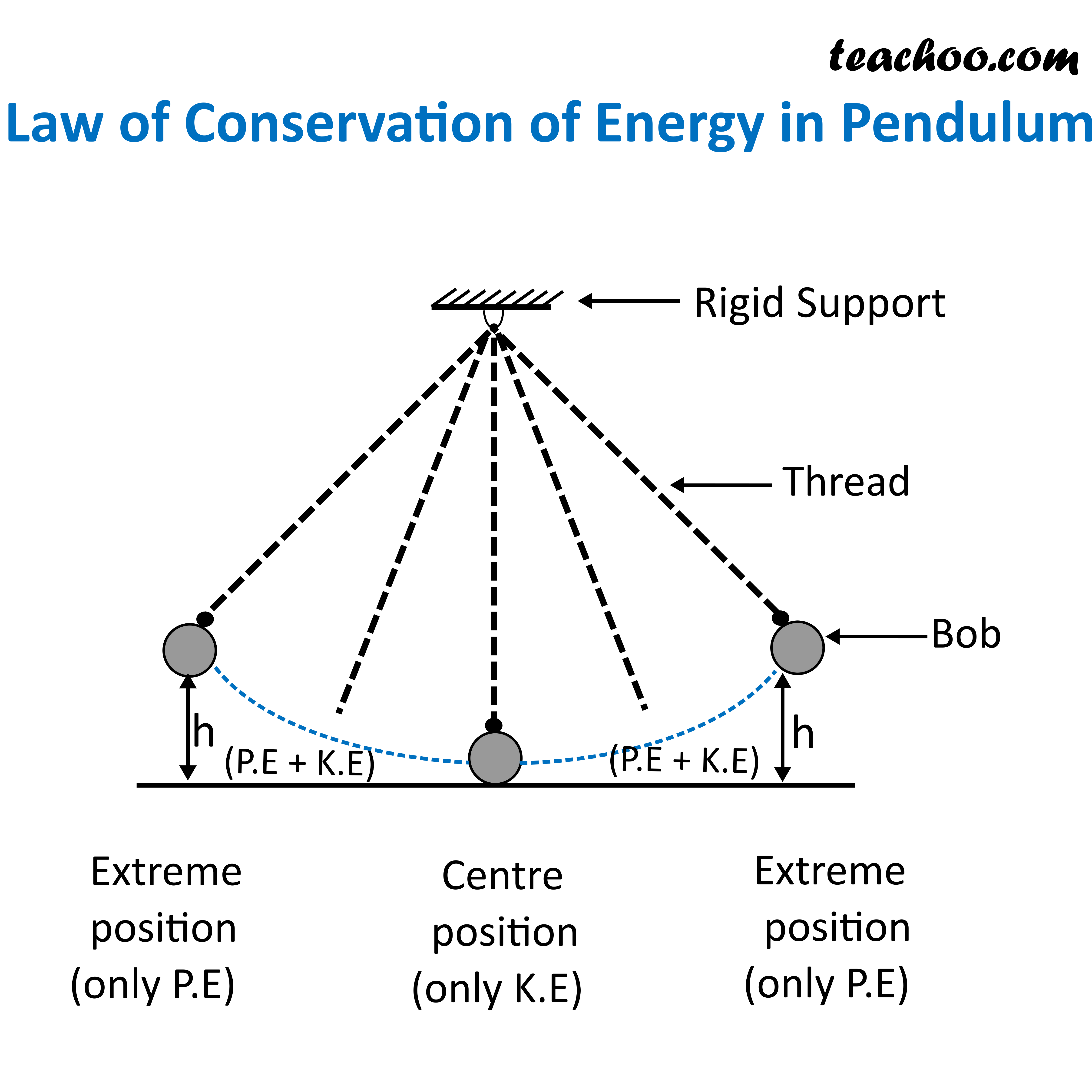
Law of Conservation of Energy with Examples Teachoo Concepts
Laws About Energy Changes The laws of thermodynamics describe how the energy in a system. A car engine burns gasoline, converting the chemical energy. The law is called the conservation of energy. Energy changes from one form of energy into another form of energy. Δ u = q − w. Laws of thermodynamics, four relations underlying thermodynamics, the branch of physics concerning heat, work, temperature,. Energy may change in form or be transferred from one. A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: There is no known exception to this law—it is exact so far as we know. The laws of thermodynamics describe how the energy in a system. Thermodynamics is the study of the relations between heat, work, temperature, and energy. The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. The law of conservation of energy states that the total energy is constant in any process. It states that there is a.
From lawofthermodynamicsinfo.com
4 Laws Of Thermodynamics With Examples (Very Simple) Laws About Energy Changes Δ u = q − w. The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: The law of conservation of energy states that. Laws About Energy Changes.
From www.youtube.com
The First Law of Thermodynamics Internal Energy, Heat, and Work YouTube Laws About Energy Changes The laws of thermodynamics describe how the energy in a system. Thermodynamics is the study of the relations between heat, work, temperature, and energy. There is no known exception to this law—it is exact so far as we know. The law of conservation of energy states that the total energy is constant in any process. The law is called the. Laws About Energy Changes.
From byjus.com
Law of Conservation of Energy Principle Of Conservation Of Energy, Derivation, Energy Laws About Energy Changes There is no known exception to this law—it is exact so far as we know. Energy may change in form or be transferred from one. The law is called the conservation of energy. Δ u = q − w. The law of conservation of energy states that the total energy is constant in any process. The laws of thermodynamics describe. Laws About Energy Changes.
From study.com
How Newton's Laws Relate to the Law of Conservation of Energy & Momentum Lesson Laws About Energy Changes A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: Thermodynamics is the study of the relations between heat, work, temperature, and energy. The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. The. Laws About Energy Changes.
From www.sliderbase.com
Energy and change Presentation Physics Laws About Energy Changes The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. Laws of thermodynamics, four relations underlying thermodynamics, the branch of physics concerning heat, work, temperature,. Energy may change in form or be transferred from one. The law of conservation of energy states. Laws About Energy Changes.
From www.sliderbase.com
Energy and change Presentation Physics Laws About Energy Changes Energy may change in form or be transferred from one. Thermodynamics is the study of the relations between heat, work, temperature, and energy. It states that there is a. A car engine burns gasoline, converting the chemical energy. The law of conservation of energy is a physical law that states that the total energy of an isolated system is a. Laws About Energy Changes.
From www.slideserve.com
PPT A Quick Review of Basic Concepts in Science, Systems, Matter, and Energy PowerPoint Laws About Energy Changes Δ u = q − w. Energy may change in form or be transferred from one. A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: Thermodynamics is the study of the relations between heat, work, temperature, and energy. The laws of thermodynamics describe how the energy in a system. A car. Laws About Energy Changes.
From www.animalia-life.club
Law Of Conservation Of Energy Equation Chemistry Laws About Energy Changes It states that there is a. Thermodynamics is the study of the relations between heat, work, temperature, and energy. There is no known exception to this law—it is exact so far as we know. A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: Δ u = q − w. The laws. Laws About Energy Changes.
From www.chemistrylearner.com
First Law of Thermodynamics Statement, Equation, & Examples Laws About Energy Changes There is no known exception to this law—it is exact so far as we know. It states that there is a. Δ u = q − w. The laws of thermodynamics describe how the energy in a system. A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: Energy changes from one. Laws About Energy Changes.
From www.totalassignmenthelp.com
A Review Of Law Of Conservation Of Energy Total Assignment Help Laws About Energy Changes A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: A car engine burns gasoline, converting the chemical energy. The law of conservation of energy states that the total energy is constant in any process. Laws of thermodynamics, four relations underlying thermodynamics, the branch of physics concerning heat, work, temperature,. It states. Laws About Energy Changes.
From chemistryguru.com.sg
How to Determine Enthalpy Change of Reaction Using Hess' Law Laws About Energy Changes The law is called the conservation of energy. Δ u = q − w. The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. It states that there is a. The law of conservation of energy states that the total energy is. Laws About Energy Changes.
From www.slideserve.com
PPT Module 01 Energy Basics Energy Power Forms of energy Thermodynamic laws Entropy / Exergy Laws About Energy Changes A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: It states that there is a. There is no known exception to this law—it is exact so far as we know. Energy may change in form or be transferred from one. Δ u = q − w. Thermodynamics is the study of. Laws About Energy Changes.
From www.pinterest.com
Law of Conservation of Energy Physics facts, Mechanical energy, Chemistry education Laws About Energy Changes Δ u = q − w. The law of conservation of energy states that the total energy is constant in any process. The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. A fundamental and commonly used formula in thermodynamics is the. Laws About Energy Changes.
From studylib.net
Energy Changes in Matter Day 1 Introduction to Chemistry and Laws About Energy Changes There is no known exception to this law—it is exact so far as we know. Energy may change in form or be transferred from one. The law of conservation of energy states that the total energy is constant in any process. A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: The. Laws About Energy Changes.
From www.grc.nasa.gov
Conservation of Energy Laws About Energy Changes Energy changes from one form of energy into another form of energy. It states that there is a. Laws of thermodynamics, four relations underlying thermodynamics, the branch of physics concerning heat, work, temperature,. A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: The law of conservation of energy is a physical. Laws About Energy Changes.
From studylib.net
ENERGY CHANGES Laws About Energy Changes A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: Energy changes from one form of energy into another form of energy. Δ u = q − w. Energy may change in form or be transferred from one. Laws of thermodynamics, four relations underlying thermodynamics, the branch of physics concerning heat, work,. Laws About Energy Changes.
From www.slideserve.com
PPT Energy Changes in Chemical Reactions Chapter 17 PowerPoint Presentation ID6596021 Laws About Energy Changes Δ u = q − w. Energy may change in form or be transferred from one. A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: Thermodynamics is the study of the relations between heat, work, temperature, and energy. The law of conservation of energy is a physical law that states that. Laws About Energy Changes.
From www.176iot.com
what is commercial unit of electrical energy converted into joules IOT Wiring Diagram Laws About Energy Changes A car engine burns gasoline, converting the chemical energy. Thermodynamics is the study of the relations between heat, work, temperature, and energy. Energy may change in form or be transferred from one. Δ u = q − w. Energy changes from one form of energy into another form of energy. A fundamental and commonly used formula in thermodynamics is the. Laws About Energy Changes.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID239015 Laws About Energy Changes It states that there is a. There is no known exception to this law—it is exact so far as we know. The laws of thermodynamics describe how the energy in a system. Energy changes from one form of energy into another form of energy. A car engine burns gasoline, converting the chemical energy. Thermodynamics is the study of the relations. Laws About Energy Changes.
From www.sliderbase.com
Energy and change Presentation Physics Laws About Energy Changes The laws of thermodynamics describe how the energy in a system. The law is called the conservation of energy. Energy may change in form or be transferred from one. Laws of thermodynamics, four relations underlying thermodynamics, the branch of physics concerning heat, work, temperature,. Thermodynamics is the study of the relations between heat, work, temperature, and energy. Energy changes from. Laws About Energy Changes.
From www.slideserve.com
PPT Energy and Matter in Ecosystems PowerPoint Presentation, free download ID5454010 Laws About Energy Changes Energy changes from one form of energy into another form of energy. There is no known exception to this law—it is exact so far as we know. A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: The law of conservation of energy is a physical law that states that the total. Laws About Energy Changes.
From www.slideserve.com
PPT Energy Laws & Types PowerPoint Presentation, free download ID3814398 Laws About Energy Changes The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. The law of conservation of energy states that the total energy is constant in any process. Energy may change in form or be transferred from one. Δ u = q − w.. Laws About Energy Changes.
From www.youtube.com
Thermodynamics 1 C2 L10 First law of thermodynamics Energy balances YouTube Laws About Energy Changes Laws of thermodynamics, four relations underlying thermodynamics, the branch of physics concerning heat, work, temperature,. It states that there is a. The law is called the conservation of energy. The laws of thermodynamics describe how the energy in a system. Δ u = q − w. The law of conservation of energy states that the total energy is constant in. Laws About Energy Changes.
From courses.lumenlearning.com
The First Law of Thermodynamics Biology for Majors I Laws About Energy Changes The laws of thermodynamics describe how the energy in a system. A car engine burns gasoline, converting the chemical energy. The law is called the conservation of energy. The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. Energy may change in. Laws About Energy Changes.
From www.youtube.com
First Law of Thermodynamics, Basic Introduction Internal Energy, Heat and Work Chemistry Laws About Energy Changes Δ u = q − w. The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. The laws of thermodynamics describe how the energy in a system. Thermodynamics is the study of the relations between heat, work, temperature, and energy. Laws of. Laws About Energy Changes.
From byjus.com
Energy Conversion & Law Of Energy Conversion with Examples Laws About Energy Changes The laws of thermodynamics describe how the energy in a system. There is no known exception to this law—it is exact so far as we know. Energy changes from one form of energy into another form of energy. It states that there is a. Energy may change in form or be transferred from one. The law is called the conservation. Laws About Energy Changes.
From www.teachoo.com
Law of Conservation of Energy with Examples Teachoo Concepts Laws About Energy Changes It states that there is a. The laws of thermodynamics describe how the energy in a system. The law of conservation of energy states that the total energy is constant in any process. A fundamental and commonly used formula in thermodynamics is the first law of thermodynamics, which is expressed as: Δ u = q − w. Thermodynamics is the. Laws About Energy Changes.
From www.slideserve.com
PPT Chapter 7 Energy and Chemical Change PowerPoint Presentation ID3203261 Laws About Energy Changes The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. A car engine burns gasoline, converting the chemical energy. There is no known exception to this law—it is exact so far as we know. Energy may change in form or be transferred. Laws About Energy Changes.
From byjus.com
Energy Conversion & Law Of Energy Conversion with Examples Laws About Energy Changes The law is called the conservation of energy. Energy changes from one form of energy into another form of energy. Energy may change in form or be transferred from one. The laws of thermodynamics describe how the energy in a system. There is no known exception to this law—it is exact so far as we know. A fundamental and commonly. Laws About Energy Changes.
From jaxson-owncreator.blogspot.com
Device That Changes Electrical Energy Into Mechanical Energy Laws About Energy Changes The law of conservation of energy states that the total energy is constant in any process. There is no known exception to this law—it is exact so far as we know. The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. The. Laws About Energy Changes.
From www.grc.nasa.gov
First Law of Thermodynamics Laws About Energy Changes A car engine burns gasoline, converting the chemical energy. Thermodynamics is the study of the relations between heat, work, temperature, and energy. There is no known exception to this law—it is exact so far as we know. The law of conservation of energy states that the total energy is constant in any process. The law of conservation of energy is. Laws About Energy Changes.
From www.engineeringstream.com
LAW OF THERMODYNAMICS Engineering Stream Laws About Energy Changes Thermodynamics is the study of the relations between heat, work, temperature, and energy. There is no known exception to this law—it is exact so far as we know. A car engine burns gasoline, converting the chemical energy. Energy may change in form or be transferred from one. Laws of thermodynamics, four relations underlying thermodynamics, the branch of physics concerning heat,. Laws About Energy Changes.
From www.electricaltechnology.org
Lenz’s Law of Induction Formula & Working Laws About Energy Changes Energy changes from one form of energy into another form of energy. A car engine burns gasoline, converting the chemical energy. The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. The law is called the conservation of energy. The law of. Laws About Energy Changes.
From byjus.com
Draw a diagram to show the energy changes in an oscillating simple pendulum. Indicate in your Laws About Energy Changes Energy changes from one form of energy into another form of energy. The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. Laws of thermodynamics, four relations underlying thermodynamics, the branch of physics concerning heat, work, temperature,. The laws of thermodynamics describe. Laws About Energy Changes.
From byjus.com
First Law of Thermodynamics Equations, Limitations and Examples Laws About Energy Changes Laws of thermodynamics, four relations underlying thermodynamics, the branch of physics concerning heat, work, temperature,. Energy changes from one form of energy into another form of energy. The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms. Thermodynamics is the study of. Laws About Energy Changes.