Table Salt Is Acidic Or Basic . determine if a salt produces an acidic or a basic solution. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? salt, in chemistry, substance produced by the reaction of an acid with a base. The prototype “salt,” of course, is sodium. the resulting ph from dissolving an acid salt in water is acidic (ph<7). A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. The ions of the salt are the conjugate base and conjugate acid of the acid and base. a salt may be defined as the product of a neutralization reaction of an acid and a base. salts can be neutral, acidic or basic. Except for their names and formulas, so far we have treated all acids as. The reaction between an acid and a base is called a neutralization reaction.
from z-cm.blogspot.com
the resulting ph from dissolving an acid salt in water is acidic (ph<7). salts can be neutral, acidic or basic. The prototype “salt,” of course, is sodium. The reaction between an acid and a base is called a neutralization reaction. why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? Except for their names and formulas, so far we have treated all acids as. salt, in chemistry, substance produced by the reaction of an acid with a base. determine if a salt produces an acidic or a basic solution. A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4).
Is Table Salt Acidic Basic Or Neutral Decoration Examples
Table Salt Is Acidic Or Basic Except for their names and formulas, so far we have treated all acids as. the resulting ph from dissolving an acid salt in water is acidic (ph<7). why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? a salt may be defined as the product of a neutralization reaction of an acid and a base. Except for their names and formulas, so far we have treated all acids as. determine if a salt produces an acidic or a basic solution. salts can be neutral, acidic or basic. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. The ions of the salt are the conjugate base and conjugate acid of the acid and base. The prototype “salt,” of course, is sodium. salt, in chemistry, substance produced by the reaction of an acid with a base. The reaction between an acid and a base is called a neutralization reaction.
From 88guru.com
What are Salts in Chemistry Characteristics, Types 88guru Table Salt Is Acidic Or Basic The ions of the salt are the conjugate base and conjugate acid of the acid and base. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). salts can be neutral, acidic or basic. salt, in chemistry, substance produced by the reaction of an acid with a base. a salt may be defined. Table Salt Is Acidic Or Basic.
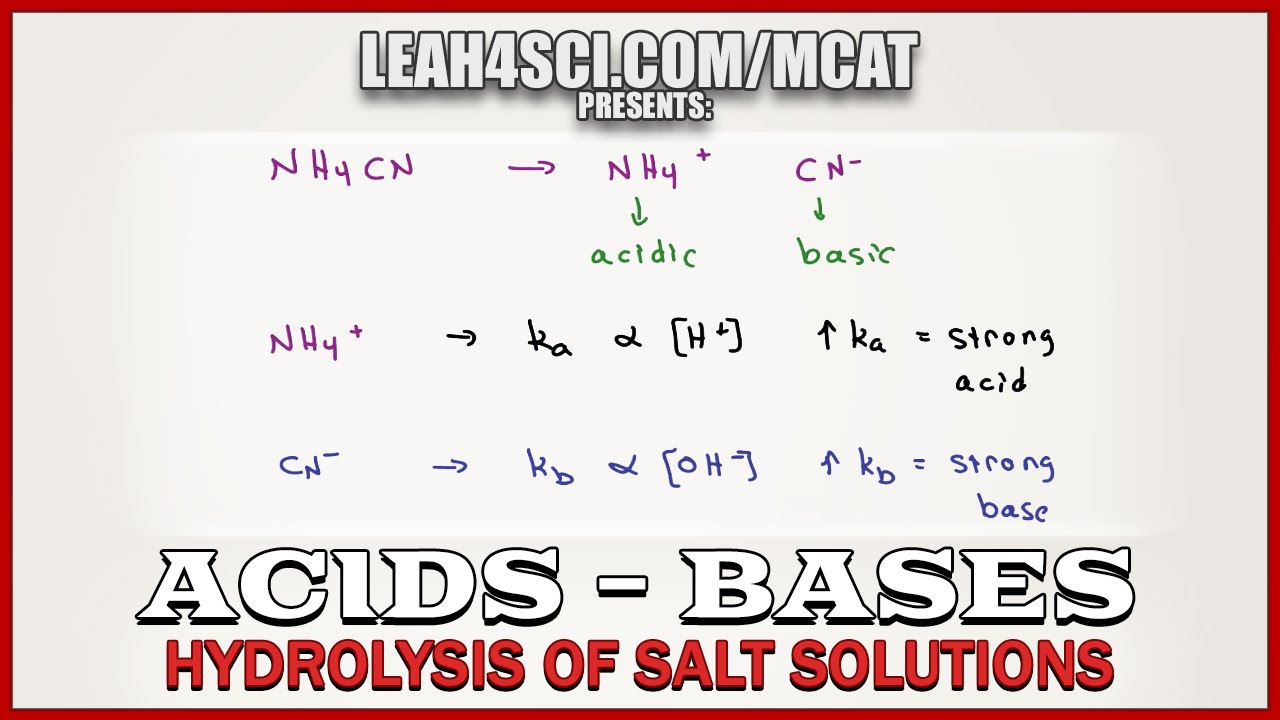
From exooredui.blob.core.windows.net
Table Salt Is Acid Or Base at Martha Hanson blog Table Salt Is Acidic Or Basic The ions of the salt are the conjugate base and conjugate acid of the acid and base. A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? Except. Table Salt Is Acidic Or Basic.
From www.sigmaaldrich.com
Acid and Base Chart — Table of Acids & Bases Table Salt Is Acidic Or Basic The prototype “salt,” of course, is sodium. salts can be neutral, acidic or basic. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). a salt may be defined as the product of a neutralization reaction of an acid and a base. The reaction between an acid and a base is called a neutralization. Table Salt Is Acidic Or Basic.
From www.teachoo.com
Salts and it's Properties (with Examples) Acids, Bases and Salt Table Salt Is Acidic Or Basic salts can be neutral, acidic or basic. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). the resulting ph from dissolving an acid salt in water is acidic (ph<7). determine if a salt produces an acidic or a basic solution. A salt consists of the positive ion (cation) of a base and. Table Salt Is Acidic Or Basic.
From www.numerade.com
SOLVED Part 1 Acidic, Basic or Neutral Salt Data and Result Table 0.1M Salt Ions Present Table Salt Is Acidic Or Basic why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? Except for their names and formulas, so far we have treated all acids as. The reaction between an acid and a base is called a neutralization reaction. the resulting ph from dissolving an acid salt in. Table Salt Is Acidic Or Basic.
From www.numerade.com
SOLVED HYDROLYSIS OF SALTS Id) sat solutions may be acidic, Name formed the salt;. basic tnat Table Salt Is Acidic Or Basic why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. determine if a salt produces an acidic or a basic solution. The reaction between an acid and. Table Salt Is Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Table Salt Is Acidic Or Basic a salt may be defined as the product of a neutralization reaction of an acid and a base. The reaction between an acid and a base is called a neutralization reaction. Except for their names and formulas, so far we have treated all acids as. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4).. Table Salt Is Acidic Or Basic.
From www.chegg.com
Solved Classify Each Salt As Acidic, Basic, Or Neutral. D... Table Salt Is Acidic Or Basic The reaction between an acid and a base is called a neutralization reaction. Except for their names and formulas, so far we have treated all acids as. why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? a salt may be defined as the product of. Table Salt Is Acidic Or Basic.
From dxofrjqld.blob.core.windows.net
What Is Acids And Bases Examples at Tyler Warner blog Table Salt Is Acidic Or Basic determine if a salt produces an acidic or a basic solution. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). a salt may be defined as the product of a neutralization reaction of an acid and a base. The prototype “salt,” of course, is sodium. the resulting ph from dissolving an acid. Table Salt Is Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Table Salt Is Acidic Or Basic Except for their names and formulas, so far we have treated all acids as. The prototype “salt,” of course, is sodium. The ions of the salt are the conjugate base and conjugate acid of the acid and base. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). determine if a salt produces an acidic. Table Salt Is Acidic Or Basic.
From www.jessicagavin.com
12 Types of Salt Every Cook Should Know Jessica Gavin Table Salt Is Acidic Or Basic Except for their names and formulas, so far we have treated all acids as. A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. determine if a salt produces an acidic or a basic solution. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). . Table Salt Is Acidic Or Basic.
From dddiqyzoeco.blob.core.windows.net
Table Salt Acidic Or Alkaline at Derek Stokes blog Table Salt Is Acidic Or Basic The prototype “salt,” of course, is sodium. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. determine if a salt produces an acidic or a basic solution. The ions of the salt are the conjugate. Table Salt Is Acidic Or Basic.
From saltsanity.com
How Much Sodium is in Table Salt? Tips for Low Sodium Living Table Salt Is Acidic Or Basic a salt may be defined as the product of a neutralization reaction of an acid and a base. salt, in chemistry, substance produced by the reaction of an acid with a base. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). A salt consists of the positive ion (cation) of a base and. Table Salt Is Acidic Or Basic.
From wizedu.com
Complete the table below and classify the following salts (acidic, basic or neutral) and their Table Salt Is Acidic Or Basic The ions of the salt are the conjugate base and conjugate acid of the acid and base. why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). determine if a salt produces. Table Salt Is Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Table Salt Is Acidic Or Basic salt, in chemistry, substance produced by the reaction of an acid with a base. why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? The prototype “salt,” of course, is sodium. A salt consists of the positive ion (cation) of a base and the negative ion. Table Salt Is Acidic Or Basic.
From www.slideserve.com
PPT Unit 6 Chpt 14&15 Acid/Base PowerPoint Presentation, free download ID4484796 Table Salt Is Acidic Or Basic the resulting ph from dissolving an acid salt in water is acidic (ph<7). salt, in chemistry, substance produced by the reaction of an acid with a base. A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. Except for their names and formulas, so far we have treated all. Table Salt Is Acidic Or Basic.
From dddiqyzoeco.blob.core.windows.net
Table Salt Acidic Or Alkaline at Derek Stokes blog Table Salt Is Acidic Or Basic salts can be neutral, acidic or basic. why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. salt, in chemistry, substance produced by the reaction of. Table Salt Is Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Table Salt Is Acidic Or Basic The prototype “salt,” of course, is sodium. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). determine if a salt produces an acidic or a basic solution. Except for their names and formulas, so far we have treated all acids as. The reaction between an acid and a base is called a neutralization reaction.. Table Salt Is Acidic Or Basic.
From www.slideserve.com
PPT ACIDS, BASES AND SALTS PowerPoint Presentation, free download ID2220893 Table Salt Is Acidic Or Basic the resulting ph from dissolving an acid salt in water is acidic (ph<7). Except for their names and formulas, so far we have treated all acids as. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). The ions of the salt are the conjugate base and conjugate acid of the acid and base. The. Table Salt Is Acidic Or Basic.
From exooredui.blob.core.windows.net
Table Salt Is Acid Or Base at Martha Hanson blog Table Salt Is Acidic Or Basic The prototype “salt,” of course, is sodium. salt, in chemistry, substance produced by the reaction of an acid with a base. The ions of the salt are the conjugate base and conjugate acid of the acid and base. A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. The reaction. Table Salt Is Acidic Or Basic.
From www.slideserve.com
PPT How are salts made and named? PowerPoint Presentation, free download ID5348558 Table Salt Is Acidic Or Basic The ions of the salt are the conjugate base and conjugate acid of the acid and base. The reaction between an acid and a base is called a neutralization reaction. Except for their names and formulas, so far we have treated all acids as. a salt may be defined as the product of a neutralization reaction of an acid. Table Salt Is Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Table Salt Is Acidic Or Basic why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? salt, in chemistry, substance produced by the reaction of an acid with a base. salts can be neutral, acidic or basic. Except for their names and formulas, so far we have treated all acids as.. Table Salt Is Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Table Salt Is Acidic Or Basic The prototype “salt,” of course, is sodium. salts can be neutral, acidic or basic. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). a salt may be defined as the product of a neutralization reaction of an acid and a base. Except for their names and formulas, so far we have treated all. Table Salt Is Acidic Or Basic.
From www.thoughtco.com
Chemical Composition of Table Salt Table Salt Is Acidic Or Basic The prototype “salt,” of course, is sodium. A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. The ions of the salt are the conjugate base and conjugate acid of the acid and base. salt, in chemistry, substance produced by the reaction of an acid with a base. An example. Table Salt Is Acidic Or Basic.
From www.youtube.com
Classifying salt solution as acidic, basic, or neutral YouTube Table Salt Is Acidic Or Basic The reaction between an acid and a base is called a neutralization reaction. determine if a salt produces an acidic or a basic solution. A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. the resulting ph from dissolving an acid salt in water is acidic (ph<7). Except for. Table Salt Is Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Table Salt Is Acidic Or Basic Except for their names and formulas, so far we have treated all acids as. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). salt, in chemistry, substance produced by the reaction of an acid with a base. A salt consists of the positive ion (cation) of a base and the negative ion (anion) of. Table Salt Is Acidic Or Basic.
From wakeup-world.com
The Health Dangers of Table Salt Wake Up World Table Salt Is Acidic Or Basic An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). the resulting ph from dissolving an acid salt in water is acidic (ph<7). The ions of the salt are the conjugate base and conjugate acid of the acid and base. salt, in chemistry, substance produced by the reaction of an acid with a base.. Table Salt Is Acidic Or Basic.
From exooredui.blob.core.windows.net
Table Salt Is Acid Or Base at Martha Hanson blog Table Salt Is Acidic Or Basic the resulting ph from dissolving an acid salt in water is acidic (ph<7). why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? a salt may be defined as the product of a neutralization reaction of an acid and a base. Except for their names. Table Salt Is Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Table Salt Is Acidic Or Basic determine if a salt produces an acidic or a basic solution. The reaction between an acid and a base is called a neutralization reaction. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). salt, in chemistry, substance produced by the reaction of an acid with a base. Except for their names and formulas,. Table Salt Is Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Table Salt Is Acidic Or Basic salts can be neutral, acidic or basic. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. why does a salt containing a cation from a weak base and an anion from a strong acid. Table Salt Is Acidic Or Basic.
From arielle-well-wolfe.blogspot.com
Chemistry Form 4 Chapter 7 Acid and Base Table Salt Is Acidic Or Basic The prototype “salt,” of course, is sodium. the resulting ph from dissolving an acid salt in water is acidic (ph<7). why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? The ions of the salt are the conjugate base and conjugate acid of the acid and. Table Salt Is Acidic Or Basic.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets, videos) Table Salt Is Acidic Or Basic The reaction between an acid and a base is called a neutralization reaction. why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? a salt may be defined as the product of a neutralization reaction of an acid and a base. salts can be neutral,. Table Salt Is Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Table Salt Is Acidic Or Basic An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). Except for their names and formulas, so far we have treated all acids as. determine if a salt produces an acidic or a basic solution. the resulting ph from dissolving an acid salt in water is acidic (ph<7). a salt may be defined. Table Salt Is Acidic Or Basic.
From www.vrogue.co
Acids Bases And Salts Igcse Chemistry Vrogue Table Salt Is Acidic Or Basic The prototype “salt,” of course, is sodium. Except for their names and formulas, so far we have treated all acids as. The reaction between an acid and a base is called a neutralization reaction. why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? a salt. Table Salt Is Acidic Or Basic.
From z-cm.blogspot.com
Is Table Salt Acidic Basic Or Neutral Decoration Examples Table Salt Is Acidic Or Basic Except for their names and formulas, so far we have treated all acids as. An example of an acid salt is sodium bisulfate (sodium hydrogen sulfate, nahso 4). why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? the resulting ph from dissolving an acid salt. Table Salt Is Acidic Or Basic.