Dilute Kmno4 Reagent . If the reaction is heated,. The correct option is c. The position directly adjacent to an aromatic group is called the “benzylic” position. Dissolve 1 gram of solid. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. kmno 4 solution should be dilute. Do you remember hydroxylation using km no4. the permanganate reagent is deep purple in color. Steps of preparation of 1% alkaline potassium permanganate solution. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids.
from www.numerade.com
Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. If the reaction is heated,. the permanganate reagent is deep purple in color. Steps of preparation of 1% alkaline potassium permanganate solution. kmno 4 solution should be dilute. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. The position directly adjacent to an aromatic group is called the “benzylic” position. Dissolve 1 gram of solid. Do you remember hydroxylation using km no4.
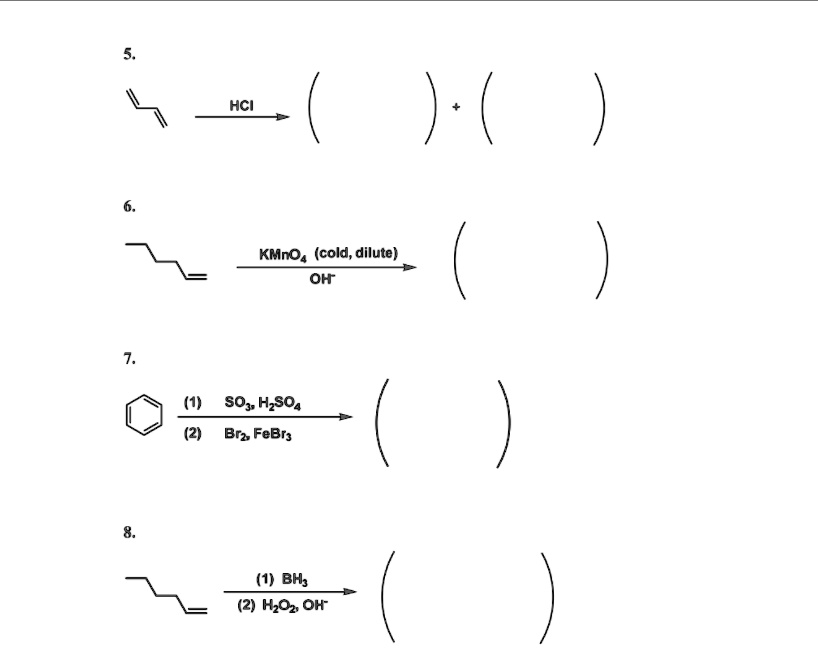
SOLVED Hci ) ( ) KMnO4 (cold, dilute) Oh ( ) (1) SO3HzSO4 Brz FeBr; BH
Dilute Kmno4 Reagent If the reaction is heated,. The position directly adjacent to an aromatic group is called the “benzylic” position. Do you remember hydroxylation using km no4. kmno 4 solution should be dilute. If the reaction is heated,. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. the permanganate reagent is deep purple in color. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. The correct option is c. Dissolve 1 gram of solid. Steps of preparation of 1% alkaline potassium permanganate solution. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids.
From www.numerade.com
SOLVED 3. Write balanced equations, naming all organic products, for Dilute Kmno4 Reagent oxidation of aromatic alkanes with kmno4 to give carboxylic acids. If the reaction is heated,. Do you remember hydroxylation using km no4. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. The correct option is c. The position directly adjacent to an aromatic group is called the “benzylic” position. Dissolve. Dilute Kmno4 Reagent.
From www.chegg.com
Solved CHO cold, dilute KMnO4, Naoh 1. hot, conc. KMnO4 2. Dilute Kmno4 Reagent kmno 4 solution should be dilute. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. Dissolve 1 gram of solid. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. Do you remember hydroxylation using km no4. the permanganate reagent is deep purple in color. Treatment of an. Dilute Kmno4 Reagent.
From www.chegg.com
Solved Select the correct reagent to get the indicated Dilute Kmno4 Reagent oxidation of aromatic alkanes with kmno4 to give carboxylic acids. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. The position directly adjacent to an aromatic group is called the “benzylic” position. If the reaction is heated,. Do you remember hydroxylation using km no4. Dissolve 1 gram of solid. Observe each. Dilute Kmno4 Reagent.
From scoop.eduncle.com
Please explain mechanism of oxidation of alkynes with hot kmno4 and Dilute Kmno4 Reagent If the reaction is heated,. The correct option is c. Dissolve 1 gram of solid. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. kmno 4 solution should be dilute. Steps of preparation of 1% alkaline potassium permanganate solution. The position directly adjacent to an aromatic group is called the “benzylic” position. Do. Dilute Kmno4 Reagent.
From mavink.com
Kmno4 Oxidation Mechanism Dilute Kmno4 Reagent kmno 4 solution should be dilute. Dissolve 1 gram of solid. the permanganate reagent is deep purple in color. Do you remember hydroxylation using km no4. The correct option is c. The position directly adjacent to an aromatic group is called the “benzylic” position. Observe each tube for the dissipation of the purple color and compare the results. Dilute Kmno4 Reagent.
From mychemblog.com
OXIDATION BY POTASSIUM PERMANGANATE (KMnO4) ALCOHOL, ALDEHYDE, ALKENE Dilute Kmno4 Reagent If the reaction is heated,. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. Steps of preparation of 1% alkaline potassium permanganate solution. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. Dissolve 1 gram of solid. Observe each tube for the dissipation of the purple color and compare the results of. Dilute Kmno4 Reagent.
From warreninstitute.org
Mastering Organic Chemistry Drawing Correct Oxidation Reaction Products Dilute Kmno4 Reagent Do you remember hydroxylation using km no4. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. The correct option is c. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. Dissolve 1 gram of. Dilute Kmno4 Reagent.
From www.numerade.com
SOLVED Hci ) ( ) KMnO4 (cold, dilute) Oh ( ) (1) SO3HzSO4 Brz FeBr; BH Dilute Kmno4 Reagent Do you remember hydroxylation using km no4. If the reaction is heated,. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. The position directly adjacent to an aromatic group is called the “benzylic” position. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. the permanganate reagent is deep purple in color.. Dilute Kmno4 Reagent.
From www.vedantu.com
Baeyer’s reagent is?A.Alkaline potassium permanganateB.Acidified Dilute Kmno4 Reagent Do you remember hydroxylation using km no4. If the reaction is heated,. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. The position directly adjacent to an aromatic group is called the “benzylic” position. kmno 4 solution should be dilute. Treatment of an alkylbenzene with potassium permanganate results in oxidation to. Dilute Kmno4 Reagent.
From www.numerade.com
SOLVEDWhich is the best reagent(s) for the following transformation Dilute Kmno4 Reagent the permanganate reagent is deep purple in color. Dissolve 1 gram of solid. Steps of preparation of 1% alkaline potassium permanganate solution. kmno 4 solution should be dilute. Do you remember hydroxylation using km no4. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. Treatment of an alkylbenzene with potassium. Dilute Kmno4 Reagent.
From www.youtube.com
Preparation and Uses of Bayers Reagent KMnO4 / OH YouTube Dilute Kmno4 Reagent The correct option is c. Dissolve 1 gram of solid. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. kmno 4 solution should be dilute. Do you remember hydroxylation using km no4. the permanganate reagent is deep purple in color. Treatment of an alkylbenzene with potassium permanganate results in oxidation. Dilute Kmno4 Reagent.
From www.chegg.com
Solved cold, dilute KMnO4 hot, conc. KMnO4 Na, NH3 NaNH2 Dilute Kmno4 Reagent The position directly adjacent to an aromatic group is called the “benzylic” position. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. the permanganate reagent is deep purple in color. If the reaction is heated,. Dissolve 1 gram of solid. Do you remember hydroxylation using km no4. Treatment of an. Dilute Kmno4 Reagent.
From www.numerade.com
SOLVED Describe the preparation of 2.10 L of 0.940 M KMnO4 from the Dilute Kmno4 Reagent the permanganate reagent is deep purple in color. The position directly adjacent to an aromatic group is called the “benzylic” position. If the reaction is heated,. Dissolve 1 gram of solid. Do you remember hydroxylation using km no4. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. Steps of preparation of 1% alkaline potassium permanganate solution. . Dilute Kmno4 Reagent.
From unacademy.com
What is Baeyer’s reagent Dilute Kmno4 Reagent Steps of preparation of 1% alkaline potassium permanganate solution. the permanganate reagent is deep purple in color. The correct option is c. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. Do you remember hydroxylation using km no4. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. kmno. Dilute Kmno4 Reagent.
From www.chemistrysteps.com
Syn Dihydroxylation of Alkenes with KMnO4 and OsO4 Chemistry Steps Dilute Kmno4 Reagent kmno 4 solution should be dilute. the permanganate reagent is deep purple in color. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. Steps of preparation of 1% alkaline potassium permanganate solution. If the reaction is heated,. The position directly adjacent to an aromatic group is called the “benzylic” position.. Dilute Kmno4 Reagent.
From www.numerade.com
SOLVED eparing Dilute KMnO4 Standard Solutions Calculate the Dilute Kmno4 Reagent Steps of preparation of 1% alkaline potassium permanganate solution. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. The correct option is c. Do you remember hydroxylation using km no4. kmno 4 solution should be dilute. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation. Dilute Kmno4 Reagent.
From www.vedantu.com
Ethylene on reaction with alkaline Kmn{O_4} givesA. GlycerolB Dilute Kmno4 Reagent Steps of preparation of 1% alkaline potassium permanganate solution. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. Do you remember hydroxylation using km no4. Dissolve 1 gram of solid. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. kmno 4 solution should be dilute. If the reaction. Dilute Kmno4 Reagent.
From www.numerade.com
SOLVED Texts Albuterol is a prescription medication for treating Dilute Kmno4 Reagent Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. kmno 4 solution should be dilute. Steps of preparation of 1% alkaline potassium permanganate solution. If the reaction is heated,. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. The correct option is c. Do. Dilute Kmno4 Reagent.
From www.numerade.com
SOLVED Redox Titration Potassium permanganate, KMnO4, is a strong Dilute Kmno4 Reagent Steps of preparation of 1% alkaline potassium permanganate solution. The correct option is c. The position directly adjacent to an aromatic group is called the “benzylic” position. the permanganate reagent is deep purple in color. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. Do you remember hydroxylation using km no4. kmno. Dilute Kmno4 Reagent.
From www.youtube.com
Baeyer's reagent Cold Dilute Alkaline KMNO4 ( Potassium Dilute Kmno4 Reagent The correct option is c. The position directly adjacent to an aromatic group is called the “benzylic” position. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. Dissolve 1 gram of solid. Do you remember hydroxylation using km no4. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. . Dilute Kmno4 Reagent.
From dxoiwwaef.blob.core.windows.net
Dilute H2So4 Mechanism at Joy Alamo blog Dilute Kmno4 Reagent The position directly adjacent to an aromatic group is called the “benzylic” position. the permanganate reagent is deep purple in color. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. kmno 4 solution should be dilute. The correct option is c. If the reaction is heated,. Dissolve 1 gram. Dilute Kmno4 Reagent.
From www.chegg.com
Solved Explain why two layers are obtained when dilute KMnO4 Dilute Kmno4 Reagent Observe each tube for the dissipation of the purple color and compare the results of the knowns with. The correct option is c. If the reaction is heated,. kmno 4 solution should be dilute. Do you remember hydroxylation using km no4. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. exhaustive oxidation. Dilute Kmno4 Reagent.
From www.coursehero.com
[Solved] . 3. Draw the following oxidation mechanism. KMnO4 HO+ dilute Dilute Kmno4 Reagent Observe each tube for the dissipation of the purple color and compare the results of the knowns with. The position directly adjacent to an aromatic group is called the “benzylic” position. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. The correct option is c. kmno 4 solution should be dilute. Do you. Dilute Kmno4 Reagent.
From ar.inspiredpencil.com
Kmno4 Mechanism Dilute Kmno4 Reagent The correct option is c. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. If the reaction is heated,. kmno 4 solution should be dilute. Dissolve 1 gram of solid. the permanganate reagent is deep purple in color. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. Do you remember. Dilute Kmno4 Reagent.
From www.transtutors.com
(Solved) Consider The Following Reaction Cold Dilute KMnO4 What Is Dilute Kmno4 Reagent Observe each tube for the dissipation of the purple color and compare the results of the knowns with. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. The position directly adjacent to an aromatic group is called the “benzylic” position. Dissolve. Dilute Kmno4 Reagent.
From www.numerade.com
SOLVED 'Draw the products of the following reactions KMnO4, Dilute Dilute Kmno4 Reagent The position directly adjacent to an aromatic group is called the “benzylic” position. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. kmno 4 solution. Dilute Kmno4 Reagent.
From www.numerade.com
SOLVED Predict the major organic product cis3hexene 1.) cold dilute Dilute Kmno4 Reagent kmno 4 solution should be dilute. Dissolve 1 gram of solid. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. Observe each tube for the. Dilute Kmno4 Reagent.
From www.chemistrysteps.com
Syn Dihydroxylation of Alkenes with KMnO4 and OsO4 Chemistry Steps Dilute Kmno4 Reagent kmno 4 solution should be dilute. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. The correct option is c. the permanganate reagent is. Dilute Kmno4 Reagent.
From www.masterorganicchemistry.com
Dihydroxylation of alkenes with cold, dilute KMnO4 to give vicinal Dilute Kmno4 Reagent The correct option is c. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. If the reaction is heated,. kmno 4 solution should be dilute. Dissolve 1 gram of solid. Do you remember. Dilute Kmno4 Reagent.
From www.masterorganicchemistry.com
Oxidation of aromatic alkanes with KMnO4 to give carboxylic acids Dilute Kmno4 Reagent Dissolve 1 gram of solid. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. the permanganate reagent is deep purple in color. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. Observe each tube for the dissipation of the purple color and compare the results. Dilute Kmno4 Reagent.
From www.ubuy.co.in
Potassium Permanganate Reagent, KMnO4, 250g, by India Ubuy Dilute Kmno4 Reagent The position directly adjacent to an aromatic group is called the “benzylic” position. Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. Do you remember hydroxylation using km no4. the permanganate reagent is deep purple in. Dilute Kmno4 Reagent.
From www.chegg.com
Solved Predict the major product AE. cold dilute KMnO4 H2 Dilute Kmno4 Reagent The position directly adjacent to an aromatic group is called the “benzylic” position. The correct option is c. Steps of preparation of 1% alkaline potassium permanganate solution. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. the permanganate reagent is deep purple in color. Treatment of an alkylbenzene with potassium. Dilute Kmno4 Reagent.
From www.youtube.com
Preparation, Standardization of 0.1N KMnO4 solution YouTube Dilute Kmno4 Reagent Dissolve 1 gram of solid. Observe each tube for the dissipation of the purple color and compare the results of the knowns with. The correct option is c. Do you remember hydroxylation using km no4. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. Steps of preparation of 1% alkaline potassium permanganate solution. kmno 4 solution should. Dilute Kmno4 Reagent.
From www.youtube.com
Alkene oxidation to vicinaldiol (cis) by cold, dilute, alkaline Dilute Kmno4 Reagent oxidation of aromatic alkanes with kmno4 to give carboxylic acids. the permanganate reagent is deep purple in color. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. If the reaction is heated,. Observe each tube for the dissipation of the purple color and compare the results of the knowns with.. Dilute Kmno4 Reagent.
From www.youtube.com
Oxidation of alkene by Baeyer s Reagent cold dilute aque. KMno4 Dilute Kmno4 Reagent the permanganate reagent is deep purple in color. oxidation of aromatic alkanes with kmno4 to give carboxylic acids. The position directly adjacent to an aromatic group is called the “benzylic” position. exhaustive oxidation of organic molecules by kmno 4 will proceed until the formation of carboxylic acids. Steps of preparation of 1% alkaline potassium permanganate solution. Dissolve. Dilute Kmno4 Reagent.