Beer's Law Nacl . The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law assumes a strictly linear dependence of the absorbance from concentration. This relationship is called the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Usually, chemical interactions and instrumental imperfection are. In other words, a solution.
from www.gpxygpfx.com
Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Beer's law assumes a strictly linear dependence of the absorbance from concentration. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Usually, chemical interactions and instrumental imperfection are. In other words, a solution.
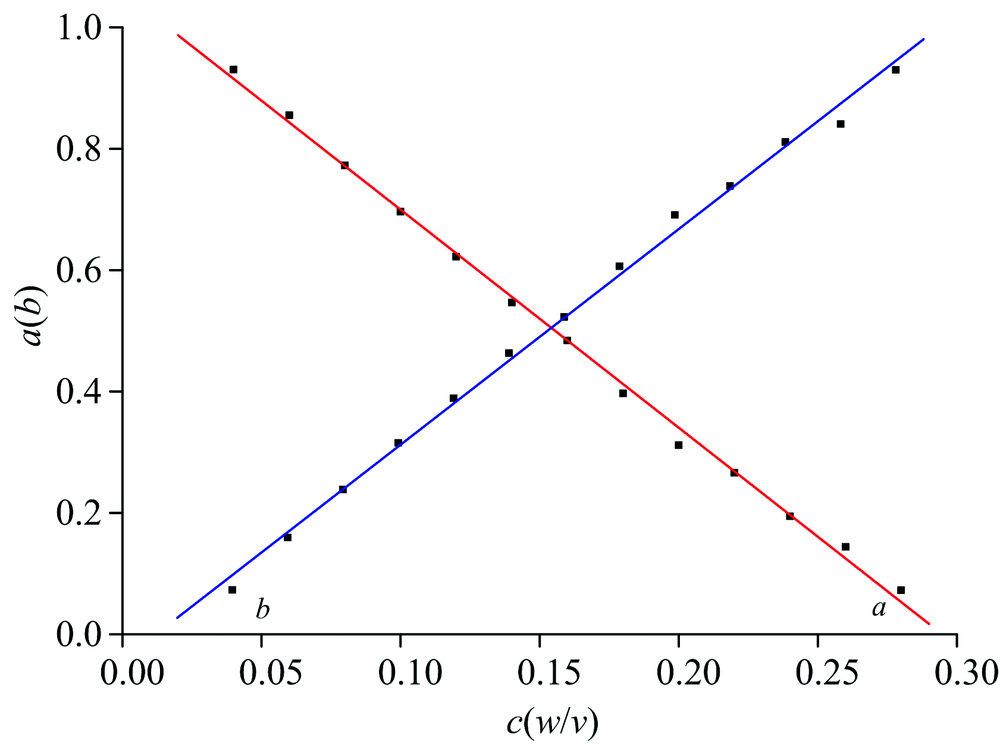
偏离朗伯比尔定律NaCl水溶液的红外光谱合成
Beer's Law Nacl Beer's law assumes a strictly linear dependence of the absorbance from concentration. In other words, a solution. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law assumes a strictly linear dependence of the absorbance from concentration. This relationship is called the. Usually, chemical interactions and instrumental imperfection are. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration.
From www.chegg.com
Solved A Beer's Law plot is shown here along with the Beer's Law Nacl Beer's law assumes a strictly linear dependence of the absorbance from concentration. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In spectroscopy, beer’s law states that. Beer's Law Nacl.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Nacl Beer's law assumes a strictly linear dependence of the absorbance from concentration. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Usually, chemical interactions and instrumental imperfection are. In spectroscopy, beer’s law states that the absorption of light by a sample is. Beer's Law Nacl.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer's Law Nacl Usually, chemical interactions and instrumental imperfection are. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and. Beer's Law Nacl.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Nacl Beer's law assumes a strictly linear dependence of the absorbance from concentration. This relationship is called the. Usually, chemical interactions and instrumental imperfection are. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In spectroscopy, beer’s law states that the absorption of light by a sample is. Beer's Law Nacl.
From www.researchgate.net
61 questions with answers in BEER LAMBERT LAW Science topic Beer's Law Nacl In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. Beer's law assumes a strictly linear dependence of the absorbance from concentration. This relationship is called the. Usually, chemical interactions and instrumental imperfection are. Since the concentration, path length and. Beer's Law Nacl.
From www.scribd.com
Beer S Law Plot of KMnO4 PDF PDF Beer's Law Nacl In other words, a solution. Usually, chemical interactions and instrumental imperfection are. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law assumes a strictly linear dependence of the absorbance from concentration. This relationship is called the. Since the concentration, path length and molar absorptivity are. Beer's Law Nacl.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Nacl In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law assumes a strictly linear dependence of the absorbance from concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Usually, chemical interactions. Beer's Law Nacl.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Nacl Usually, chemical interactions and instrumental imperfection are. This relationship is called the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law assumes a strictly linear dependence of the absorbance from concentration. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively. Beer's Law Nacl.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer's Law Nacl In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. This relationship is called the. Beer's law assumes a strictly linear dependence of the absorbance from concentration. In other words, a solution. The amount of light that a species absorbs in a spectroscopic transition can. Beer's Law Nacl.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Nacl Beer's law assumes a strictly linear dependence of the absorbance from concentration. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. Usually, chemical interactions and instrumental imperfection are. This relationship is called the. Since the concentration, path length and. Beer's Law Nacl.
From studylib.net
Exp 2 Beer's Law Beer's Law Nacl In other words, a solution. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Usually, chemical interactions and instrumental imperfection are. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's. Beer's Law Nacl.
From www.numerade.com
SOLVED Could a Beer’s Law experiment accurately determine the Beer's Law Nacl The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Usually, chemical interactions and instrumental imperfection are. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In spectroscopy, beer’s law states that the absorption of light by. Beer's Law Nacl.
From app.formative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Beer's Law Nacl This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In other words, a solution. Beer's law assumes a strictly linear dependence of the absorbance from concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we. Beer's Law Nacl.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Nacl The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. Since the concentration, path length and molar absorptivity. Beer's Law Nacl.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Nacl This relationship is called the. In other words, a solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law assumes a strictly linear dependence of. Beer's Law Nacl.
From www.researchgate.net
Beer's law plot of second derivative data [HMBATSC] = 1 x 103 M Beer's Law Nacl In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In other words, a solution. Usually, chemical interactions and instrumental imperfection are. The amount of. Beer's Law Nacl.
From chem.libretexts.org
8.2 Beer's Law Chemistry LibreTexts Beer's Law Nacl The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law assumes a strictly linear dependence of the absorbance from concentration. Usually,. Beer's Law Nacl.
From www.studocu.com
Chem 181 4 Beer's Law Beer Lab Pre lab Determining the Beer's Law Nacl Beer's law assumes a strictly linear dependence of the absorbance from concentration. This relationship is called the. Usually, chemical interactions and instrumental imperfection are. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The amount of light that a species absorbs in a spectroscopic. Beer's Law Nacl.
From www.slideserve.com
PPT CHM 5175 Part 2.3 PowerPoint Presentation, free download ID Beer's Law Nacl This relationship is called the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In other words, a solution. Usually, chemical interactions and instrumental imperfection are. Beer's. Beer's Law Nacl.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Nacl Beer's law assumes a strictly linear dependence of the absorbance from concentration. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since. Beer's Law Nacl.
From www.gpxygpfx.com
偏离朗伯比尔定律NaCl水溶液的红外光谱合成 Beer's Law Nacl In other words, a solution. Usually, chemical interactions and instrumental imperfection are. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law assumes a strictly linear dependence of the absorbance from concentration. In spectroscopy, beer’s law states that the absorption of light by a sample is. Beer's Law Nacl.
From www.researchgate.net
Fig. S1. Beer's law calibration curve for the UVVis spectrum of Beer's Law Nacl This relationship is called the. Beer's law assumes a strictly linear dependence of the absorbance from concentration. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In. Beer's Law Nacl.
From www.slideserve.com
PPT Beers Law for a Single Component Sample PowerPoint Presentation Beer's Law Nacl Beer's law assumes a strictly linear dependence of the absorbance from concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In other words, a solution. Usually,. Beer's Law Nacl.
From www.researchgate.net
Beer's law curves for method A and method B Download Scientific Diagram Beer's Law Nacl This relationship is called the. Beer's law assumes a strictly linear dependence of the absorbance from concentration. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its. Beer's Law Nacl.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Nacl Usually, chemical interactions and instrumental imperfection are. Beer's law assumes a strictly linear dependence of the absorbance from concentration. This relationship is called the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional. Beer's Law Nacl.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Nacl The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Usually, chemical interactions and instrumental imperfection are. Beer's law assumes a strictly linear dependence of the absorbance from concentration. In other words, a solution. Since the concentration, path length and molar absorptivity are all directly proportional to the. Beer's Law Nacl.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law Nacl Beer's law assumes a strictly linear dependence of the absorbance from concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In other words, a solution. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This. Beer's Law Nacl.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Nacl In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer's Law Nacl.
From chemistrysources.com
قانون بيير (بير) Beer’s Law التعريف و المعادلة مصادر الكيمياء Beer's Law Nacl Usually, chemical interactions and instrumental imperfection are. Beer's law assumes a strictly linear dependence of the absorbance from concentration. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. This relationship is called the. The amount of light that a. Beer's Law Nacl.
From www.researchgate.net
Fig. S3. Beer's law calibration curve for the UVVis spectrum of Beer's Law Nacl In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law assumes a strictly linear dependence of the absorbance from concentration. In. Beer's Law Nacl.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Nacl In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. This relationship is called the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Usually, chemical interactions and instrumental imperfection are. Beer's law assumes. Beer's Law Nacl.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer's Law Nacl Usually, chemical interactions and instrumental imperfection are. In other words, a solution. This relationship is called the. Beer's law assumes a strictly linear dependence of the absorbance from concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The amount of light that a species absorbs in a spectroscopic. Beer's Law Nacl.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Nacl The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Usually, chemical interactions and instrumental imperfection are. Beer's law assumes a strictly linear dependence of the absorbance from concentration. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is. Beer's Law Nacl.
From www.numerade.com
SOLVED 2 Practice using Beer's Law to determine the concentration of a Beer's Law Nacl Beer's law assumes a strictly linear dependence of the absorbance from concentration. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Usually,. Beer's Law Nacl.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Nacl The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In other words, a solution. Usually, chemical interactions and instrumental imperfection are. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In spectroscopy, beer’s law states that. Beer's Law Nacl.