Liquid Bromine Reacts With Solid Phosphorus . Consider the reaction of solid phosphorous with liquid bromine: 2 p (s) + 5 br 2(l) 2. Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. The reaction between phosphorus and liquid bromine is outlined below. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); Reaction of bromine with phosphorus. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Suppose 2.63 g of phosphorous reacts. Most simply, using white phosphorus:
from www.numerade.com
Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); The reaction between phosphorus and liquid bromine is outlined below. 2 p (s) + 5 br 2(l) 2. Suppose 2.63 g of phosphorous reacts. Most simply, using white phosphorus: Consider the reaction of solid phosphorous with liquid bromine: In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide.
SOLVEDPart III Mass Relationships in Chemical Reactions (Stoichiometry
Liquid Bromine Reacts With Solid Phosphorus Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); Most simply, using white phosphorus: The reaction between phosphorus and liquid bromine is outlined below. Suppose 2.63 g of phosphorous reacts. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. Consider the reaction of solid phosphorous with liquid bromine: 2 p (s) + 5 br 2(l) 2. Reaction of bromine with phosphorus.
From www.chegg.com
Solved Red phosphorus, a solid, reacts exothermically with Liquid Bromine Reacts With Solid Phosphorus 2 p (s) + 5 br 2(l) 2. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); Most simply, using white phosphorus: In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. Consider the reaction of solid phosphorous with liquid bromine: Reaction. Liquid Bromine Reacts With Solid Phosphorus.
From www.youtube.com
Phosphorus Reacts with Bromine Periodic Table of Videos YouTube Liquid Bromine Reacts With Solid Phosphorus Reaction of bromine with phosphorus. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Consider the reaction of solid phosphorous with liquid bromine: Most simply, using white phosphorus: Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) +. Liquid Bromine Reacts With Solid Phosphorus.
From www.numerade.com
SOLVEDPart III Mass Relationships in Chemical Reactions (Stoichiometry Liquid Bromine Reacts With Solid Phosphorus Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); Suppose 2.63 g of phosphorous reacts. 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. 2 p (s) + 5 br 2(l) 2. In an excess of chlorine or bromine, phosphorus reacts to form. Liquid Bromine Reacts With Solid Phosphorus.
From courses.lumenlearning.com
Classifying Chemical Reactions Chemistry Atoms First Liquid Bromine Reacts With Solid Phosphorus Most simply, using white phosphorus: In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); The reaction between phosphorus and liquid bromine is outlined below. 2 p (s) + 5 br 2(l) 2.. Liquid Bromine Reacts With Solid Phosphorus.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Liquid Bromine Reacts With Solid Phosphorus Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Reaction of bromine with phosphorus. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. The reaction between phosphorus and liquid bromine is outlined below. 2 p (s) + 5 br 2(l) 2. 2 p (s) + 3 br_2 (l). Liquid Bromine Reacts With Solid Phosphorus.
From www.youtube.com
Red phosphorus reacts with liquid bromine in an exothermic reaction 2P Liquid Bromine Reacts With Solid Phosphorus Reaction of bromine with phosphorus. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. Most simply, using white phosphorus: 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. Suppose 2.63 g of phosphorous reacts.. Liquid Bromine Reacts With Solid Phosphorus.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Liquid Bromine Reacts With Solid Phosphorus Most simply, using white phosphorus: Reaction of bromine with phosphorus. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the. Liquid Bromine Reacts With Solid Phosphorus.
From askfilo.com
Red phosphorus reacts with liquid bromine in exothermic reaction as follo.. Liquid Bromine Reacts With Solid Phosphorus In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. 2 p (s) + 5 br 2(l) 2. The reaction between phosphorus and liquid bromine is outlined below. Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. 2 p. Liquid Bromine Reacts With Solid Phosphorus.
From www.toppr.com
2. Red phosphorus reacts with liquid bromine as 2P(s) + 3Bry (1) 2PBrz Liquid Bromine Reacts With Solid Phosphorus Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); Consider the reaction of solid phosphorous with liquid bromine: 2 p (s) + 5 br 2(l) 2. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid. Liquid Bromine Reacts With Solid Phosphorus.
From www.youtube.com
Reaction between red phosphorus and liquid bromine is an exothermic Liquid Bromine Reacts With Solid Phosphorus 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or. Liquid Bromine Reacts With Solid Phosphorus.
From www.doubtnut.com
Red phosphorus reacts with liquid bromine in an exotermic reaction 2 Liquid Bromine Reacts With Solid Phosphorus Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. The reaction between phosphorus and liquid bromine is outlined below. Most simply, using white phosphorus: 2 p (s) + 5 br 2(l) 2. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Reaction of bromine with phosphorus. Reaction between red phosphorus and liquid bromine is. Liquid Bromine Reacts With Solid Phosphorus.
From www.chegg.com
Solved Phosphorus and bromine react to form phosphorus Liquid Bromine Reacts With Solid Phosphorus In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. 2 p (s) + 5 br 2(l) 2. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); Suppose 2.63 g of phosphorous reacts. Reaction of bromine with phosphorus. Most simply, using white. Liquid Bromine Reacts With Solid Phosphorus.
From www.toppr.com
CHCOUCH) HOVOX Red phosphorus reacts with liquid bromine as 2P(s) + 3Br Liquid Bromine Reacts With Solid Phosphorus The reaction between phosphorus and liquid bromine is outlined below. Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. 2 p (s) + 5 br 2(l) 2. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s). Liquid Bromine Reacts With Solid Phosphorus.
From fphoto.photoshelter.com
bromine phases transition chemistry element Fundamental Photographs Liquid Bromine Reacts With Solid Phosphorus Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Consider the reaction of solid phosphorous with liquid bromine: 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. Reaction of bromine with phosphorus. Most simply, using white phosphorus: In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v). Liquid Bromine Reacts With Solid Phosphorus.
From www.toppr.com
2. Red phosphorus reacts with liquid bromine as 2P(s) + 3Bry (1) 2PBrz Liquid Bromine Reacts With Solid Phosphorus Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); The reaction between phosphorus and liquid bromine is outlined below. Most simply, using white phosphorus: 2 p (s) + 5 br 2(l) 2. 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. In an. Liquid Bromine Reacts With Solid Phosphorus.
From www.chegg.com
Solved Answer Requested PartB Liquid bromine reacts with Liquid Bromine Reacts With Solid Phosphorus Suppose 2.63 g of phosphorous reacts. Reaction of bromine with phosphorus. 2 p (s) + 5 br 2(l) 2. Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Consider the reaction of solid phosphorous with liquid bromine: In an excess of chlorine or bromine, phosphorus. Liquid Bromine Reacts With Solid Phosphorus.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID2440715 Liquid Bromine Reacts With Solid Phosphorus Suppose 2.63 g of phosphorous reacts. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. The reaction between phosphorus and liquid bromine is outlined below. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Consider the reaction of solid phosphorous with liquid bromine: Phosphorous reacts with excess br. Liquid Bromine Reacts With Solid Phosphorus.
From www.meritnation.com
In the given image from NCERT textbook , in the equation there is Liquid Bromine Reacts With Solid Phosphorus Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); The reaction between phosphorus and liquid bromine is outlined below. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. 2 p (s). Liquid Bromine Reacts With Solid Phosphorus.
From byjus.com
How do you test for an alkene? Liquid Bromine Reacts With Solid Phosphorus Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Reaction of bromine with phosphorus. Most simply, using white phosphorus: Consider the reaction of solid phosphorous with liquid bromine: 2 p (s) + 5 br 2(l) 2. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. 2 p (s) + 3 br_2 (l). Liquid Bromine Reacts With Solid Phosphorus.
From www.youtube.com
Red phosphorus reacts with liquid bromine in an exotermic reaction 2P Liquid Bromine Reacts With Solid Phosphorus 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. Most simply, using white phosphorus: Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. 2 p (s) + 5 br 2(l) 2. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. In an excess of chlorine or bromine, phosphorus reacts. Liquid Bromine Reacts With Solid Phosphorus.
From www.sciencephoto.com
Phosphorus and Bromine Reacting (1 of 3) Stock Image C003/3530 Liquid Bromine Reacts With Solid Phosphorus Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Most simply, using white phosphorus: 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. 2. Liquid Bromine Reacts With Solid Phosphorus.
From www.chegg.com
Solved Part B Bromine liquid reacts with aqueous potassium Liquid Bromine Reacts With Solid Phosphorus Most simply, using white phosphorus: Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. The reaction between phosphorus and liquid bromine is outlined below. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); Suppose 2.63 g of phosphorous reacts. In an excess of chlorine or bromine, phosphorus. Liquid Bromine Reacts With Solid Phosphorus.
From www.sciencephoto.com
Phosphorus and Bromine Reacting (3 of 3) Stock Image C003/3466 Liquid Bromine Reacts With Solid Phosphorus Most simply, using white phosphorus: Reaction of bromine with phosphorus. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. The reaction between phosphorus and liquid bromine is outlined below. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows. Liquid Bromine Reacts With Solid Phosphorus.
From www.chegg.com
1.Use the following information to answer the next Liquid Bromine Reacts With Solid Phosphorus The reaction between phosphorus and liquid bromine is outlined below. Suppose 2.63 g of phosphorous reacts. Reaction of bromine with phosphorus. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Reaction. Liquid Bromine Reacts With Solid Phosphorus.
From www.youtube.com
How to Balance P + Br2 =PBr3 (Phosphorous + Bromine gas) YouTube Liquid Bromine Reacts With Solid Phosphorus 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. Consider the reaction of solid phosphorous with liquid bromine: Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Reaction of bromine with phosphorus. The reaction between phosphorus and liquid bromine is outlined below. Reaction between red phosphorus and liquid bromine is. Liquid Bromine Reacts With Solid Phosphorus.
From ar.inspiredpencil.com
Bromine Liquid Formula Liquid Bromine Reacts With Solid Phosphorus Suppose 2.63 g of phosphorous reacts. Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); 2 p (s) + 5 br 2(l) 2. Consider the reaction of solid phosphorous with liquid bromine: 2 p (s) + 3 br_2. Liquid Bromine Reacts With Solid Phosphorus.
From www.coursehero.com
[Solved] The mechanism shows how a solution of bromine in water reacts Liquid Bromine Reacts With Solid Phosphorus Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. Reaction of bromine with phosphorus. The reaction between phosphorus and liquid bromine is outlined below. 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. Reaction. Liquid Bromine Reacts With Solid Phosphorus.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Liquid Bromine Reacts With Solid Phosphorus In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. The reaction between phosphorus and liquid bromine is outlined below. 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. Most simply, using white phosphorus: Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Suppose. Liquid Bromine Reacts With Solid Phosphorus.
From www.chegg.com
Solved Q. Phosphorus (P) and liquid bromine (Br2).3 react Liquid Bromine Reacts With Solid Phosphorus 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); Consider. Liquid Bromine Reacts With Solid Phosphorus.
From www.dreamstime.com
A Chemical Reaction To Produce Liquid Bromine in a Beaker, in the Liquid Bromine Reacts With Solid Phosphorus The reaction between phosphorus and liquid bromine is outlined below. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Consider the reaction of solid phosphorous with liquid bromine: Most simply, using white phosphorus: Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented. Liquid Bromine Reacts With Solid Phosphorus.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Liquid Bromine Reacts With Solid Phosphorus Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. 2 p (s) + 5 br 2(l) 2. Consider the reaction of solid phosphorous with liquid bromine: Most simply, using white phosphorus: Suppose 2.63 g of phosphorous reacts. Reaction of bromine with phosphorus. 2. Liquid Bromine Reacts With Solid Phosphorus.
From kunduz.com
[ANSWERED] 1 Solid aluminum metal reacts with liquid bromine giving Liquid Bromine Reacts With Solid Phosphorus Reaction of bromine with phosphorus. Consider the reaction of solid phosphorous with liquid bromine: Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. 2 p (s) + 5 br 2(l) 2. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Most simply, using white phosphorus: 2 p (s) + 3 br_2 (l) rightarrow 2. Liquid Bromine Reacts With Solid Phosphorus.
From www.livescience.com
Facts About Bromine Live Science Liquid Bromine Reacts With Solid Phosphorus Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. Liquid bromine reacts with solid phosphorus (p_4) (p 4) to produce solid diphosphorus pentabromide. Reaction of bromine with phosphorus. 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. 2 p (s) + 5 br 2(l) 2. Reaction between red phosphorus and liquid bromine is an. Liquid Bromine Reacts With Solid Phosphorus.
From www.sciencephoto.com
Phosphorus and Bromine Reacting (2 of 3) Stock Image C003/3529 Liquid Bromine Reacts With Solid Phosphorus Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. 2 p (s) + 5 br 2(l) 2. The reaction between phosphorus and liquid bromine is outlined below. Most simply, using white phosphorus: Consider the reaction of solid phosphorous with liquid bromine: 2 p (s) + 3 br_2 (l) rightarrow 2 pbr_3 (l) identify the limiting. Liquid bromine reacts with. Liquid Bromine Reacts With Solid Phosphorus.
From www.numerade.com
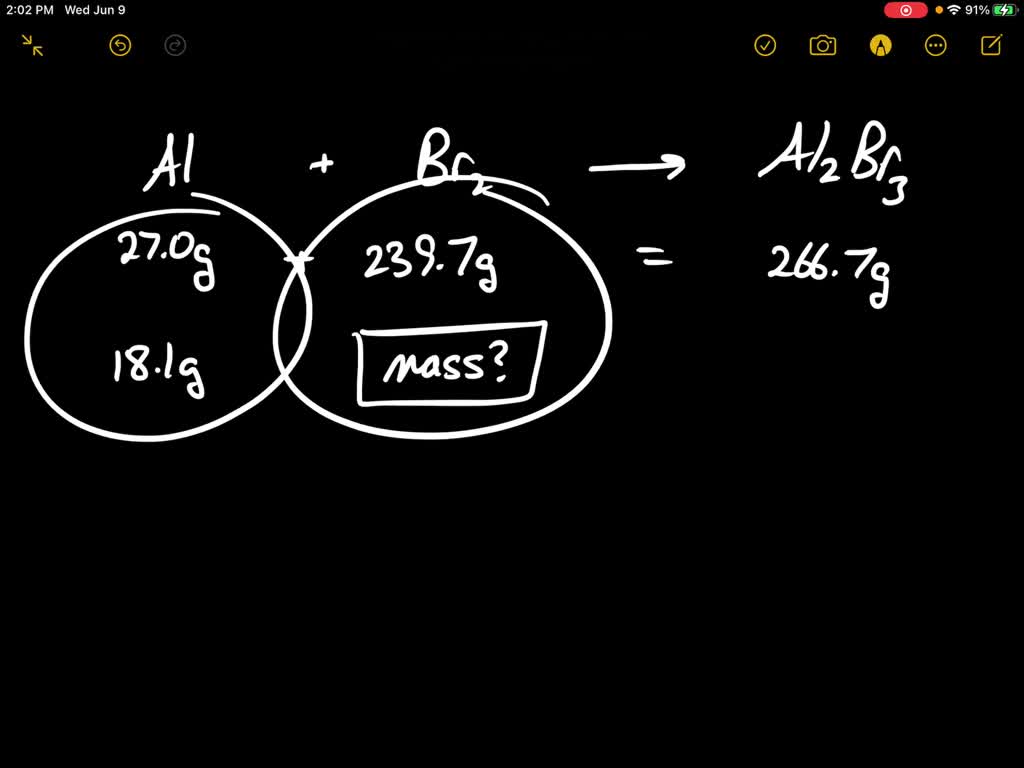
SOLVED Solid aluminum metal and diatomic bromine liquid react Liquid Bromine Reacts With Solid Phosphorus Phosphorous reacts with excess br 2 forming phosphorous (v)chloride [4]. The reaction between phosphorus and liquid bromine is outlined below. In an excess of chlorine or bromine, phosphorus reacts to form phosphorus (v) chloride or bromide. Reaction between red phosphorus and liquid bromine is an exothermic reaction represented as follows 2p (s) + 3br2 (l) 2pbr3 (g); Suppose 2.63 g. Liquid Bromine Reacts With Solid Phosphorus.