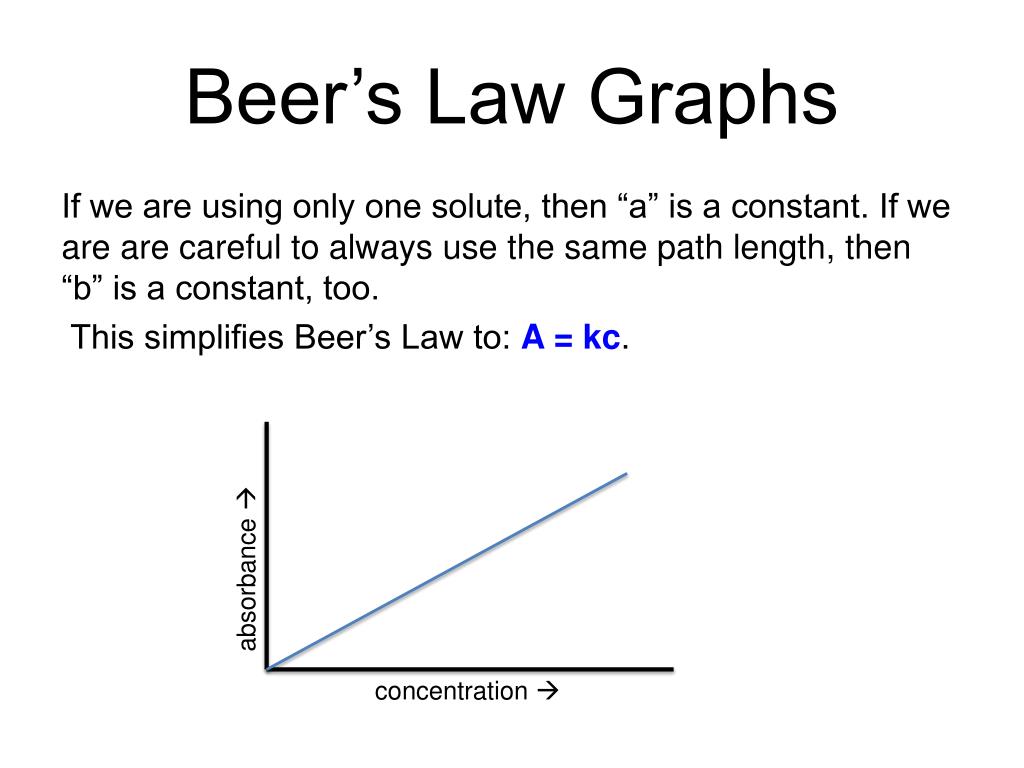
Beer's Law Used . beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. fundamental limitations to beer's law. Beer’s law is a limiting law that is valid only for low concentrations of analyte. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. There are two contributions to this.
from www.slideserve.com
Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. fundamental limitations to beer's law. There are two contributions to this.
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download
Beer's Law Used since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. There are two contributions to this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. fundamental limitations to beer's law. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law is a limiting law that is valid only for low concentrations of analyte.
From openlab.citytech.cuny.edu
Beer’s Law Biology 1101 Course Hub Beer's Law Used beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. fundamental limitations to beer's law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. There are two contributions to this. Beer’s law is a limiting law that is valid only. Beer's Law Used.
From www.scribd.com
Application of Beers Law Beer's Law Used There are two contributions to this. Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. . Beer's Law Used.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Used There are two contributions to this. Beer’s law is a limiting law that is valid only for low concentrations of analyte. fundamental limitations to beer's law. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law Used.
From public.iorodeo.com
Lab 2 Beer’s Law and Molar Extinction Coefficients — Colorimeter User Beer's Law Used There are two contributions to this. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. fundamental limitations to beer's law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law is a limiting law that is valid only. Beer's Law Used.
From www.youtube.com
Help with Beer's Law Lab Calculations YouTube Beer's Law Used There are two contributions to this. fundamental limitations to beer's law. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law is a limiting law that is valid only. Beer's Law Used.
From lelandchemclub.weebly.com
Leland Chemistry Club Leland Chemistry Club News Beer's Law Used Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. There are two contributions to this. . Beer's Law Used.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation ID2407366 Beer's Law Used There are two contributions to this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. fundamental limitations to beer's law. Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in spectroscopy, a relation concerning the absorption of radiant. Beer's Law Used.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Used There are two contributions to this. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. fundamental limitations to beer's law. Beer’s law is a limiting law that is valid only. Beer's Law Used.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law Used since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. fundamental limitations to beer's law. Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There. Beer's Law Used.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Used fundamental limitations to beer's law. There are two contributions to this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in spectroscopy, a relation concerning the absorption of radiant. Beer's Law Used.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Used since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law is a limiting law that is valid only for low concentrations of analyte. There are two contributions to this. fundamental limitations to beer's law. beer’s law, in spectroscopy, a relation concerning the absorption of radiant. Beer's Law Used.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Used beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law is a limiting law that is valid only for low concentrations of analyte. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. There are two contributions to this. . Beer's Law Used.
From dxottmlor.blob.core.windows.net
Chemical Equation For Brewing Beer at Mark Willingham blog Beer's Law Used Beer’s law is a limiting law that is valid only for low concentrations of analyte. fundamental limitations to beer's law. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There are two contributions to this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law Used.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Used Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. There are two contributions to this. . Beer's Law Used.
From thechemistrynotes.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer's Law Used fundamental limitations to beer's law. Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. There. Beer's Law Used.
From www.slideserve.com
PPT Beer’s Law and Spectrophotometry PowerPoint Presentation ID211563 Beer's Law Used fundamental limitations to beer's law. There are two contributions to this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law is a limiting law that is valid only. Beer's Law Used.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer's Law Used Beer’s law is a limiting law that is valid only for low concentrations of analyte. fundamental limitations to beer's law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There. Beer's Law Used.
From www.youtube.com
Beer's Law Concept, Calculations, & Lab YouTube Beer's Law Used Beer’s law is a limiting law that is valid only for low concentrations of analyte. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There are two contributions to this. . Beer's Law Used.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Used Beer’s law is a limiting law that is valid only for low concentrations of analyte. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. fundamental limitations to beer's law. There are two contributions to this. beer’s law, in spectroscopy, a relation concerning the absorption of radiant. Beer's Law Used.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID795528 Beer's Law Used There are two contributions to this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law is a limiting law that is valid only for low concentrations of analyte. . Beer's Law Used.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Used beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. There are two contributions to this. Beer’s law is a limiting law that is valid only for low concentrations of analyte. . Beer's Law Used.
From www.slideserve.com
PPT Chemical the rates of reactions PowerPoint Presentation Beer's Law Used fundamental limitations to beer's law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There. Beer's Law Used.
From www.scribd.com
beers law lab report example Molar Concentration Concentration Beer's Law Used since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. There are two contributions to this. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law is a limiting law that is valid only for low concentrations of analyte. . Beer's Law Used.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Used There are two contributions to this. fundamental limitations to beer's law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in spectroscopy, a relation concerning the absorption of radiant. Beer's Law Used.
From app.formative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Beer's Law Used fundamental limitations to beer's law. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There are two contributions to this. Beer’s law is a limiting law that is valid only for low concentrations of analyte. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law Used.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Beer's Law Used Beer’s law is a limiting law that is valid only for low concentrations of analyte. fundamental limitations to beer's law. There are two contributions to this. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law Used.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Used since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There are two contributions to this. Beer’s law is a limiting law that is valid only for low concentrations of analyte. . Beer's Law Used.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Used beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There are two contributions to this. Beer’s law is a limiting law that is valid only for low concentrations of analyte. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. . Beer's Law Used.
From chemistrysources.com
قانون بيير (بير) Beer's Law التعريف و المعادلة مصادر الكيمياء Beer's Law Used There are two contributions to this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. fundamental limitations to beer's law. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law is a limiting law that is valid only. Beer's Law Used.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Used Beer’s law is a limiting law that is valid only for low concentrations of analyte. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. fundamental limitations to beer's law. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There. Beer's Law Used.
From studylib.net
Beer`s Law Beer's Law Used fundamental limitations to beer's law. Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There are two contributions to this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law Used.
From www.chegg.com
Solved Beer's Law is applied most accurately when a Beer's Law Used fundamental limitations to beer's law. There are two contributions to this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in spectroscopy, a relation concerning the absorption of radiant. Beer's Law Used.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Beer's Law Used since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. There are two contributions to this. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. fundamental limitations to beer's law. Beer’s law is a limiting law that is valid only. Beer's Law Used.
From www.slideshare.net
Beers law Beer's Law Used fundamental limitations to beer's law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There are two contributions to this. Beer’s law is a limiting law that is valid only. Beer's Law Used.
From www.learnatnoon.com
What are some Limitations of Beer Lambert Law? Noon Academy Beer's Law Used since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. There are two contributions to this. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law is a limiting law that is valid only for low concentrations of analyte. . Beer's Law Used.